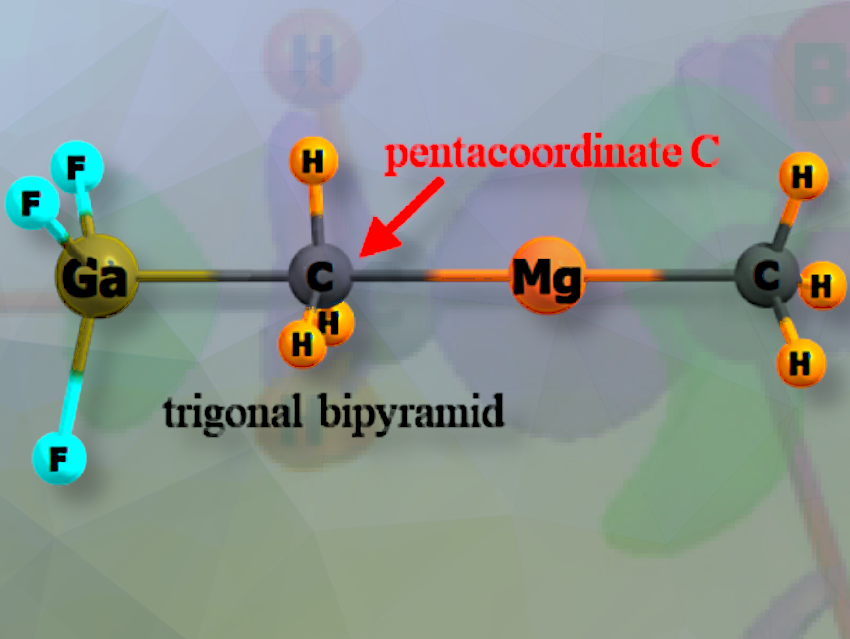
Steve Scheiner, Utah State University, Logan, USA, and colleagues have studied whether methyl groups in Be and Mg complexes can act as electron donors in triel bonds with B, Al, or Ga, and provide the first evidence of a pentacoordinate carbon in a stable complex.
A triel bond (TrB) is a type of non-covalent interaction in which a triel atom (a Group 13 element) accepts electron density from a donor atom or molecule, often through a π hole, which is a region of electron deficiency on the triel atom. This interaction, which is similar to hydrogen bonding, is important for understanding the bonding properties of materials and chemical systems containing these elements.
What did you do?
This work addresses whether a methyl group can act as an electron donor in a triel bond with a B, Al, or Ga atom. The methyl group was placed on an alkaline earth Be or Mg metal atom to increase the availability of its electron density. Quantum chemical calculations revealed that some of these triel bonds are quite strong, with interaction energies approaching 50 kcal/mol.
Why are you interested in this?
The triel bond is probably the least explored of the new family of non-covalent bonds that are parallel to the H-bond. And the question of whether a methyl group can participate in these bonds raises some important issues with potential applications to numerous fields.
What is new and cool about your research?
Some of these complexes contained a high degree of transfer of the methyl group from the metal to the triel atom. Methyl transfers are important to a set of enzymes with biological relevance.
Also of some importance is the finding that as part of its transfer, the C atom of the methyl group takes on a pentacoordinate geometry, similar in shape to the SN2 transition state, but here contained within a stable complex. This is the first such observation of this phenomenon.
What is the main significance of your results?
The ability of the methyl group to transfer between these particular atoms is a new finding, as is the pentacoordinate trigonal bipyramid shape of the complex with the C atom lying in the center.
What is the longer-term vision for your research?
The ability of the methyl group to transfer between these particular atoms broadens our understanding of this process which may lead to new applications in enzymology, catalysis, and macromolecular structure.
What part of your work was the most challenging?
There were some difficulties in isolating certain geometries and distinguishing their characteristics as true minima on the potential energy surface.
In computational chemistry, identifying a true minimum on the potential energy surface means confirming that a molecular geometry corresponds to a stable, low-energy configuration rather than a transition state or unstable structure. This requires ensuring that the geometry has no imaginary frequencies, which can be challenging for complex systems with multiple configurations close in energy.
Thank you very much for these insights.
The paper they talked about:
- Triel Bonds with Methyl Groups as Electron Donors. A Pentacoordinate Carbon Atom,
Xin Wang, Yuwei Cheng, Qingzhong Li, Steve Scheiner,
ChemPhysChem 2024.
https://doi.org/10.1002/cphc.202400931

Steve Scheiner is a Professor in the Chemistry and Biochemistry Department of Utah State University in Logan, USA.