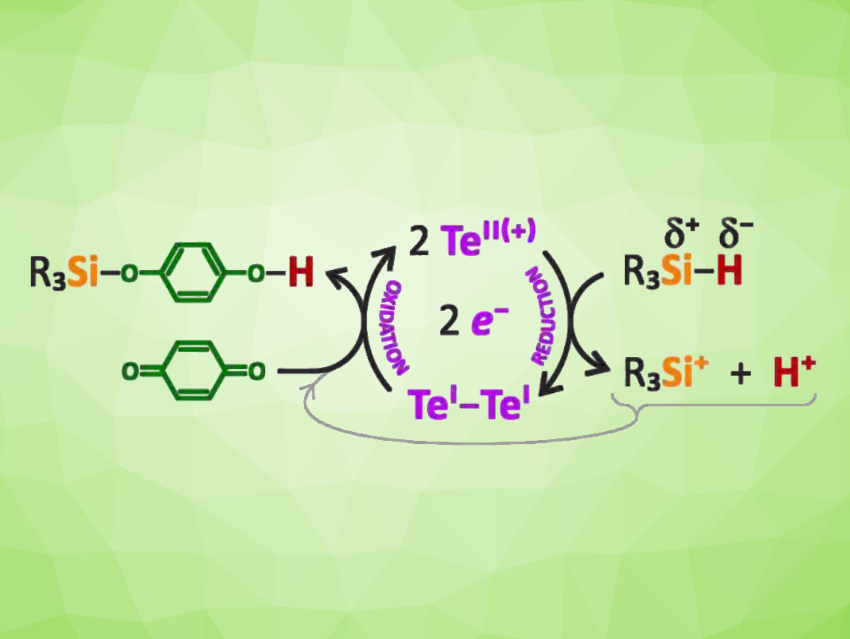
Originally, redox catalysis tended to be restricted to transition metals, but in recent years, there has been progress in developing catalytic redox cycles mediated by main group elements. Elements such as phosphorus and bismuth can be useful in this context because they have multiple oxidation states of comparable stability. This is also true for some group 16 elements such as tellurium. However, the utilization of elements such as tellurium for redox catalysis, e.g., for bond activation chemistry, has been rare so far.
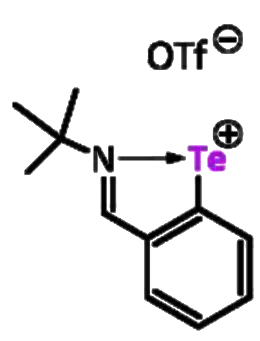 Martin Hejda, University of Pardubice, Czech Republic, Emanuel Hupf, Jens Beckmann, University of Bremen, Germany, and colleagues have found a tellurium compound that can be used as a main group catalyst. The team used the C,N-chelated aryltellurenyl triflate [2-(tBuNCH)C6H4Te][OTf] (pictured on the right, OTf– = triflate)- The tellurenyl cation can be considered a Lewis superacidic carbene analogue, as “naked” tellurenyl cations of the type [RTe]+ are electron-deficient species with six valence electrons.
Martin Hejda, University of Pardubice, Czech Republic, Emanuel Hupf, Jens Beckmann, University of Bremen, Germany, and colleagues have found a tellurium compound that can be used as a main group catalyst. The team used the C,N-chelated aryltellurenyl triflate [2-(tBuNCH)C6H4Te][OTf] (pictured on the right, OTf– = triflate)- The tellurenyl cation can be considered a Lewis superacidic carbene analogue, as “naked” tellurenyl cations of the type [RTe]+ are electron-deficient species with six valence electrons.
The researchers used this catalyst for Si–H bond activations in different tertiary silanes of the type R3SiH (R = Et, Ph, TMS, with TMS = Si(CH3)3) to give the iminium salts (tBuN(H)CH)C6H4TeSiR3][OTf]. This bond activation can be utilized in main group redox catalysis, e.g., to convert p-quinones into silylated hydroquinones (general reaction pictured at the top).
- Redox Cycling with Tellurium. Si–H Bond Activation by a Lewis Superacidic Tellurenyl Cation,
Martin Hejda, Emanuel Hupf, Aleš Růžička, Libor Dostál, Jens Beckmann,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202403496