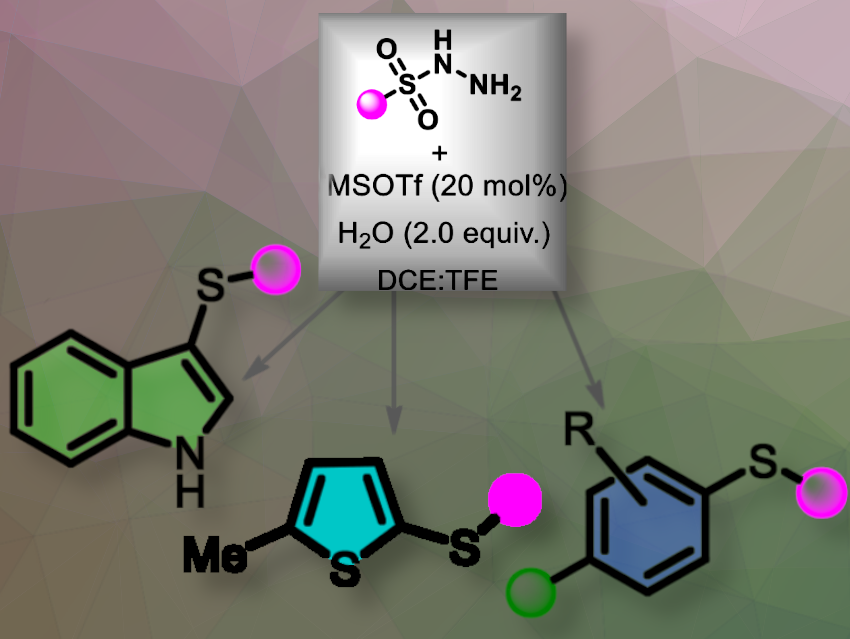
Rahul Vishwakarma, Pragya Sharma, and Chinmoy Kumar Hazra, Indian Institute of Technology Delhi, have developed a catalytic system that enables the regioselective thiolation of electron-rich arenes and heteroarenes with sulfonyl hydrazides, yielding para-thio-substituted products efficiently.
In their one-pot reaction, the team used the silylating acent trimethylsilyl trifluoromethanesulfonate (TMS•OTf) as the active catalyst, generating Brønsted acid in situ upon contact with water. They used sulfonyl hydrazides as environmentally friendly sulfur sources. The reaction was erfomed in a trifluoroethanol (TFE) and 1,2-dichloroethane (DCE) solvent mixture at 80 °C. The reactions were conducted using tosylhydrazide and 1,3,5-trimethoxybenzene as model substrates. This method efficiently produces para-thio-substituted arenes and 3-sulfenyl-indoles in good to excellent yields.
The researchers think their reaction is significance in forming C–S bonds found in various biologically active compounds.
- Lewis acid-promoted C–H Chalcogenation of Arenes and Heteroarenes,
Rahul Vishwakarma, Pragya Sharma, Chinmoy Kumar Hazra,
Europ. J. Org. Chem. 2024.
https://doi.org/10.1002/ejoc.202400894