Plant Fungus Provides New Drug With A New Cellular Target
Novel chemical compounds from a fungus could provide new perspectives for treating colorectal cancer, one of the most common and deadliest cancers worldwide. Ninghua Tan, Yi Ma, Zhe Wang, and colleagues, China Pharmaceutical University, Nanjing, China, have reported on the isolation and characterization of a previously unknown class of metabolites (terpene-nonadride heterodimers). One of these compounds effectively kills colorectal cancer cells by attacking the enzyme DCTPP1, which thus may serve as a potential biomarker for colorectal cancer and a therapeutic target.
Challenges in Colorectal Cancer Treatment
Rather than using conventional cytostatic drugs, which have many side effects, modern cancer treatment frequently involves targeted tumor therapies directed at specific target molecules in the tumor cells. However, the prognosis for colorectal cancer patients remains grim—there is a need for new targets and novel drugs.
Targeted tumor therapies are mostly based on small molecules from plants, fungi, bacteria, and marine organisms. About half of current cancer medications were developed from natural substances. The researchers chose to use Bipolaris victoriae S27, a fungus that lives on plants, as the starting point in their search for new drugs.
Discovery of Novel Metabolites
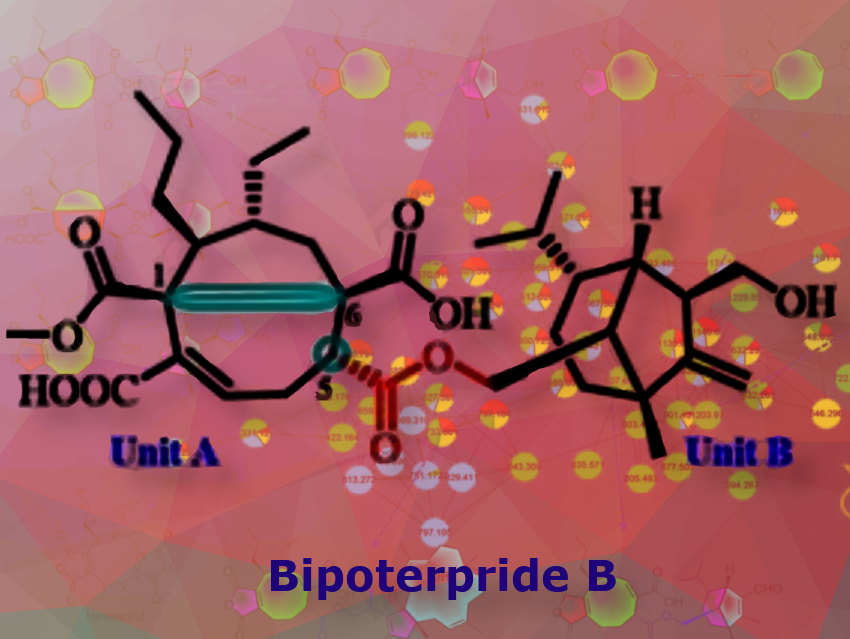
The team first analyzed metabolic products by cultivating the fungus under many different conditions (OSMAC method, one strain, many compounds). They discovered twelve unusual chemical structures belonging to a previously unknown class of compounds: terpene-nonadride heterodimers, molecules made from one terpene and one nonadride unit.
Widely found in nature, terpenes are a large group of compounds with very varied carbon frameworks based on isoprene units. Nonadrides are nine-membered carbon rings with maleic anhydride groups. The monomers making up this class of dimers termed “bipoterprides” were also identified and were found to contain additional structural novelties (bicyclic 5/6-nonadrides with carbon rearrangements).
Nine of the bipoterprides were effective against colorectal cancer cells. The most effective was bipoterpride No. 2, which killed tumor cells as effectively as the classic cytostatic drug Cisplatin. In mouse models, it caused tumors to shrink with no toxic side effects.
Mechanism of Action
The team used a variety of methods to analyze the drug’s mechanism: bipoterpride 2 inhibits dCTP-pyrophosphatase 1 (DCTPP1), an enzyme that regulates the cellular nucleotide pool. The heterodimer binds significantly more tightly than each of its individual monomers. The activity of DCTPP1 is elevated in certain types of tumors, promoting the invasion, migration, and proliferation of the cancer cells while also inhibiting programmed cell death. It can also help cancer cells to resist treatment. Bipoterpride 2 inhibits this enzymatic activity and disrupts the—pathologically altered—amino acid metabolism in the tumor cells.
The team was thus able to identify DCTPP1 as a new target for the treatment of colorectal cancer and bipoterprides as new potential drug candidates.
- Identification of Novel Target DCTPP1 for Colorectal Cancer Therapy with the Natural Small-Molecule Inhibitors Regulating Metabolic Reprogramming,
Li Feng, Xinjia Wang, Xinrui Guo, Liyuan Shi, Shihuang Su, Xinjing Li, Jia Wang, Prof. Ninghua Tan, Yi Ma, Zhe Wang,
Angewandte Chemie International Edition 2024.
https://doi.org/10.1002/anie.202402543