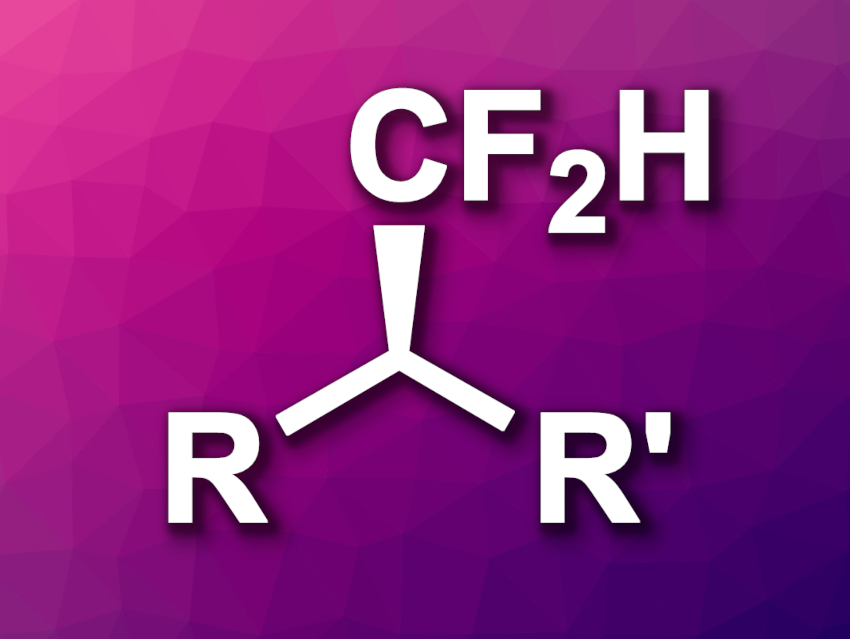
The introduction of fluorine atoms into organic compounds can be useful, e.g., in pharmaceutical chemistry to tune the properties of drug candidates. Difluoromethyl (CF2H) groups are often used in this context. Methods for the introduction of these groups, especially in an enantioselective manner, are useful.
Wei Liu, University of Cincinnati, Ohio, USA, and colleagues have developed a method for the nickel-catalyzed decarboxylative difluoromethylation of alkyl carboxylic acids that gives difluoromethylated products with high enantioselectivities. The team used (DMPU)2Zn(CF2H)2 as a source of CF2H units (DMPU = N,N’-dimethylpropyleneurea), Ni(ClO4)2·6H2O as the catalyst together with a chiral bisoxazoline ligand, [Ir(ppy)2(dtbbpy)(PF6)] (dtbbpy = 2,2′-bis(4-tert-butylpyridine, ppy = phenylpyridine) as a photocatalyst, Mg(OAc)2 and formic acid as additives, and tetrahydrofuran (THF) as the solvent. The reactions were performed under blue LED light at –10 °C.
Under these conditions, the desired difluoromethylated products were obtained under loss of CO2 in mostly moderate to good yields and with excellent enantioselectivities. The team proposes a reaction mechanism that involves the formation of an alkyl radical from the carboxylic acid under CO2 loss, mediated by the iridium photocatalyst, followed by the enantioselective addition of the CF2H unit via a chiral NiII-CF2H complex. Overall, the method shows a good functional group tolerance and can provide access to a wide variety of fluorinated derivatives of bioactive compounds.
- Highly Enantioselective Decarboxylative Difluoromethylation,
Xian Zhao, Chao Wang, Lingfeng Yin, Wei Liu,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c11257