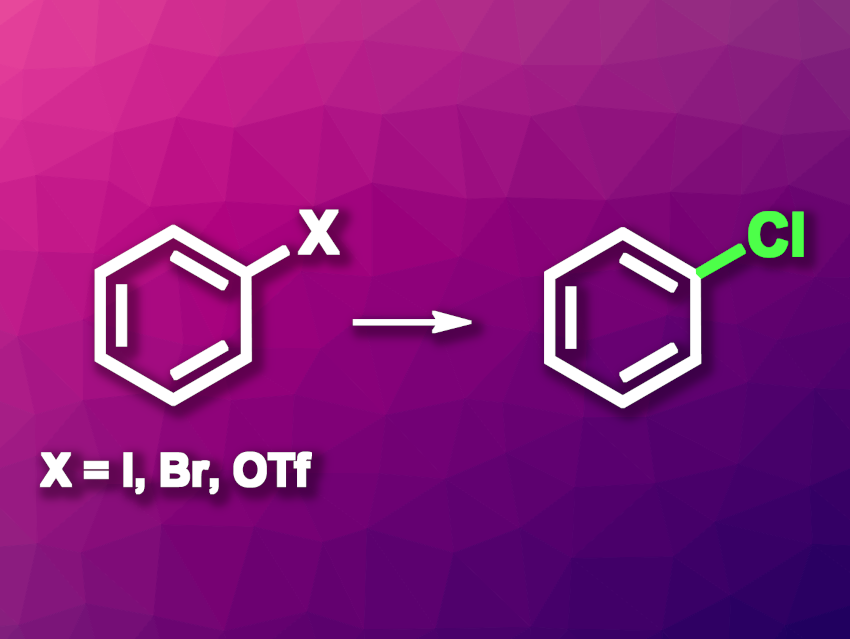
Aryl halides are widely used in organic synthesis, for example, in transition-metal-catalyzed cross-coupling reactions. Exchanging different halides can be useful to tune reactivity, e.g., for late-stage transformations. Converting aryl bromides or aryl iodides to aryl chlorides can prevent unwanted reactions. However, existing methods for this type of transformation can suffer from drawbacks such as a need for high temperatures or oxidative reaction conditions.
Yu-Feng Liang, Shandong University, Jinan, China, and colleagues have developed a method for the chlorination of aryl halides and triflates under mild conditions (pictured, OTf– = triflate). The team reacted a variety of aryl bromides, iodides, and triflates with MgCl, using NiCl2 as a catalyst, zinc as a reductant, and dimethylacetamide (DMA) as the solvent. The reactions were performed at room temperature.
Under these conditions, the desired aryl chlorides were obtained in mostly good to excellent yields. The team proposes a reaction mechanism in which the Ni(II) is first reduced to Ni(0) by the zinc. An oxidative addition to the aryl halide then gives a Ni(II) intermediate of the type Ar–Ni–X. This is followed by the halogen exchange reaction with MgCl2. According to the researchers, the Ni(II) intermediate is usually not well-suited to reductive elimination, but the magnesium may facilitate this step by lowering its energy barrier, leading to the formation of the desired aryl chlorides. Overall, the developed method allows for a chlorination of aryl halides and triflates under mild reaction conditions and with good functional group tolerance.
- Magnesium-promoted nickel-catalysed chlorination of aryl halides and triflates under mild conditions,
Tian-Yu Zhang, Muhammad Bilal, Tian-Zhang Wang, Chao-Peng Zhang, Yu-Feng Liang,
Chem. Commun. 2024.
https://doi.org/10.1039/D4CC04383A