Thomas Martin Lowry was born on October 26, 1874, in Low Moor, UK, as the son of a Methodist minister [1,2]. He went to Kingswood School, a private boarding school in Bath, UK, and then to Central Technical College in London, UK, an educational institution set up by the City of London and guilds, or “livery companies”, to promote technical education.
Lowry showed an interest in chemistry and studied under Henry Edward Armstrong. He served as Armstrong’s assistant from 1896 to 1913 and completed his Ph.D. in 1899. Starting in 1904, he simultaneously was a Lecturer at Westminster Training College in London, a higher education institute and teacher training college founded by the Methodist Church.
In 1913, Lowry was appointed Head of the chemical department at the Medical School of Guy’s Hospital, a teaching hospital in London, and also was named a Professor at the University of London. Starting in 1920, he served as the Chair of Physical Chemistry at the University of Cambridge, UK, a position he held until his passing on November 2, 1936.
Lowry was a Founding Member of the Faraday Society and served as its President from 1928 to 1930. He was elected a fellow of the UK’s Royal Society in 1914. He was awarded the Order of the British Empire and the Italian Order of Saints Maurice and Lazarus for his services during World War I in connection with work on filling shells with an explosive mixture [3].
Lowry is best known for coining the term “mutarotation” and the devlopment of the Brønsted–Lowry acid–base theory. You can read more about his work below.
Mutarotation
During his time with Henry Edward Armstrong, Lowry made important contributions to organic chemistry. In 1898/99, while working on the chemistry of terpenes, he observed a change in the optical rotation of nitrocamphor over time [4].
In 1903, Lowry coined the term “mutarotation” to describe this phenomenon in a context where it is particularly well-known: the interconversion of the anomeric forms of glucose [5]. Cyclic forms of sugars show mutarotation as their α- and β-anomeric forms, which have different specific optical rotations, interconvert in solution (see example in Fig. 1). When a solution is freshly prepared, this leads to a change in the overall optical rotation of the solution until the equilibrium between the different forms is reached.
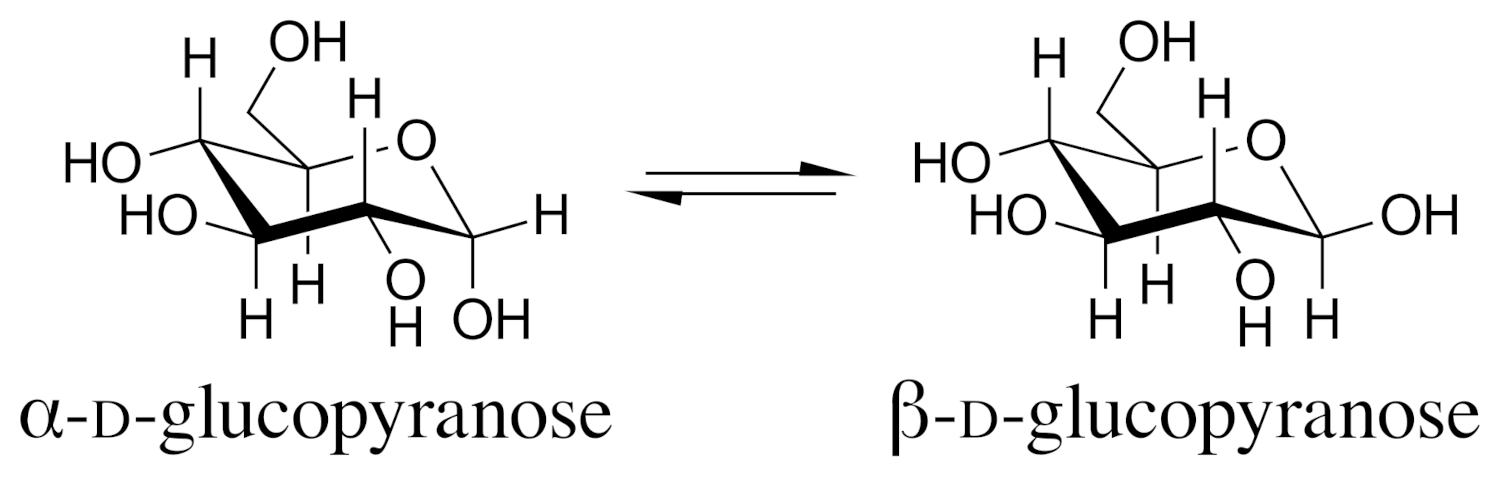 |
|
Figure 1. α- and β-D-glucopyranose anomers (wikimedia commons, Vaccinationist, CC BY-SA 4.0) |
What Are Acids and Bases, Exactly?
The first definition of molecular acids and bases in modern chemistry was devised by Svante Arrhenius. His theory of dissociation, which states that molecules of acids, bases, and salts dissociate into ions when dissolved in water, was honored with the Nobel Prize in Chemistry in 1903.
Arrhenius extended this theory to devise definitions for acids and bases: According to him, acids are substances that produce hydrogen ions (H+) in solution, and bases are substances that produce hydroxide ions (OH–) in solution. These definitions are restricted to aqueous solutions. For other solvents, a new definition was, thus, needed.
Lowry developed a theory that accounts for solvents other than water simultaneously with, and independently of, the Danish chemist Johannes Nicolaus Brønsted in 1923 [6,7]. The definition of acids and bases put forth by both Lowry and Brønsted in the “Brønsted–Lowry acid–base theory” is that acids are proton donators and bases are proton acceptors, or, as Lowry puts it in his publication on the topic [6], “A base is best described as an acceptor of hydrogen nuclei.” This definition avoids a “need” for the presence of water to form hydroxide ions.
In the Brønsted–Lowry acid–base theory, the focus is not on a solute/salt and a solvent (such as water), but on pairs of conjugate acids and conjugate bases that are formed in an acid–base reaction from the base and the acid, respectively (see Eq. 1).
|
Equation 1. HA: acid, B: base, BH+: conjugate acid of B, A–: conjugate base of HA. |
This definition also accounts for amphoteric compounds that can act as either an acid or a base depending on the reaction partner—such as water, which can act as an acid and donate a proton to give its conjugated base OH–, but can also act as a base and accept a proton to give its conjugated acid, the hydronium ion H3O+ (see Eq. 2 for an example).
|
Equation 2. Water can act as both acid and base, as shown here in its autoprotolysis reaction. |
Even with this extended definition, some compounds that “act acidic” in solution, such as AlCl3, were not included because they cannot donate a proton. They are, however, included in the Lewis definition of acid–base reactions, which was devised by Gilbert N. Lewis [8].
Lewis defines an acid as an electron-pair acceptor and a base as an electron-pair donor. For example, the base NH3 has a lone electron pair it can “donate”, while the acid BF3 has an electron sextet around the boron atoms, i.e., it is short of an electron octet and can “accept” an electron pair. Compounds such as these two can form a Lewis acid–base adduct with a dative bond (i.e., in this case, H3N–BF3). The different acid–base definitions of Arrhenius, Brønsted and Lowry, and Lewis become increasingly more general in that order.
References
[1] Thomas Martin Lowry, 1874–1936,
William Jackson Pope,
Biogr. Mem. Fellows R. Soc. 1938, 2, 286–293.
https://doi.org/10.1098/rsbm.1938.0009
[2] Obituary notices: John Kenneth Harold Inglis, 1877–1935; Thomas Martin Lowry, 1874–1936; Camille Matignon, 1867–1934; Julius Arthur Nieuwland, 1878–1936; P. A. Ellis Richards, 1868–1936; Percy Richard Sanders, 1875–1937,
N. T. M. Wilsmore, W. J. Pope, W. S. Calcott, F. W. Edwards, A. More,
J. Chem. Soc. 1927, 700–711.
https://doi.org/10.1039/JR9370000700
[3] History and the teaching of chemistry. A tribute to Thomas Lowry’s textbook “Historical Introduction to Chemistry”,
William B. Jensen,
Educ. Quim. 2016, 27, 175–181.
https://doi.org/10.1016/j.eq.2016.05.001
[4] XXVI. — Studies of the Terpenes and Allied Compounds. Nitrocamphor and its Derivatives. IV. Nitrocamphor as an Example of Dynamic Isomerism,
T. Martin Lowry,
J. Chem. Soc. Trans. 1899, 75, 211–244.
[5] CXXV.—Studies of dynamic isomerism. I. The mutarotation of glucose,
T. Martin Lowry,
J. Chem. Soc. Trans. 1903, 83, 1314–1323.
https://doi.org/10.1039/CT9038301314
[6] The uniqueness of hydrogen,
T. Martin Lowry,
J. Chem. Chem. Ind. 1923, 42, 43–47.
https://doi.org/10.1002/jctb.5000420302
[7] Einige Bemerkungen über den Begriff der Säuren und Basen,
J. N. Brønsted,
Recl. Trav. Chim. Pays-Bas 1923, 42, 718–728.
https://doi.org/10.1002/recl.19230420815
[8] Acids and bases,
G. N. Lewis,
J. Franklin Inst. 1938, 226, 293–313.
https://doi.org/10.1016/S0016-0032(38)91691-6
Also of Interest
Physical Chemistry Pioneer: Svante Arrhenius (1859 – 1927),
ChemistryViews 2023.
https://doi.org/10.1002/chemv.202300014
Swedish Nobel Laureate known for work on the theory of electrolytic dissociation, activation energy, and the pioneering theory of global warming



Love to read and study more of it,
GRK