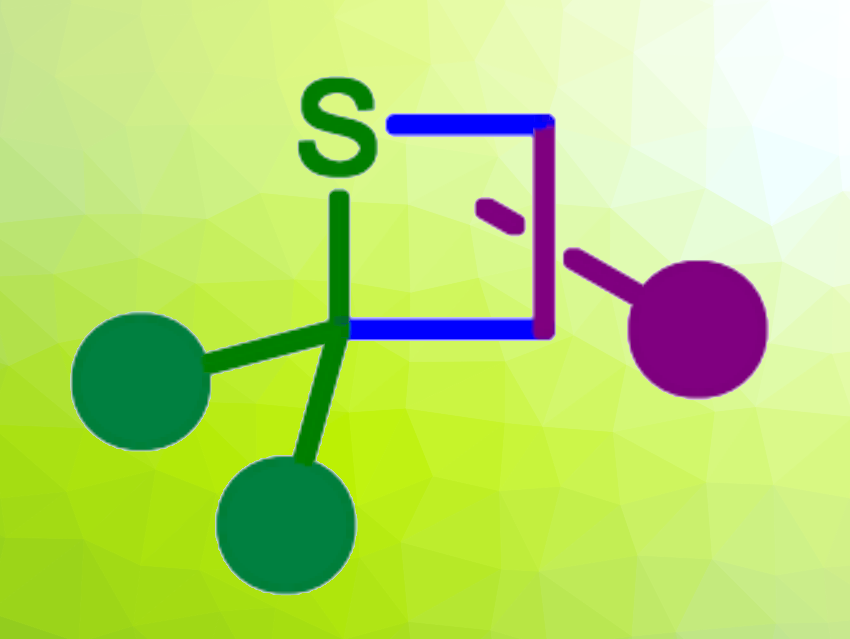
The thia-Paternò-Büchi reaction is a [2+2]-photocycloaddition that forms four-membered rings with a sulfur atom, i.e., thietanes (pictured above) from thiocarbonyl compounds and alkenes. Thiocarbonyl compounds can be unstable, which has hampered the use of this type of reaction—in particular, for thioketones and thioaldehydes.
Thomas Boddaert, Université Paris-Saclay, CNRS, Orsay, France, and colleagues have developed a domino reaction that can work around this limitation. The team’s approach is based on a Norrish type II fragmentation of pyrenacyl sulfides (pictured below), which generates thiocarbonyls in situ, followed by the thia-Paternò-Büchi reaction. The team found that the 1-acetylpyrene also generated in situ during the fragmentation acts as the photocatalyst for the thia-Paternò-Büchi reaction.
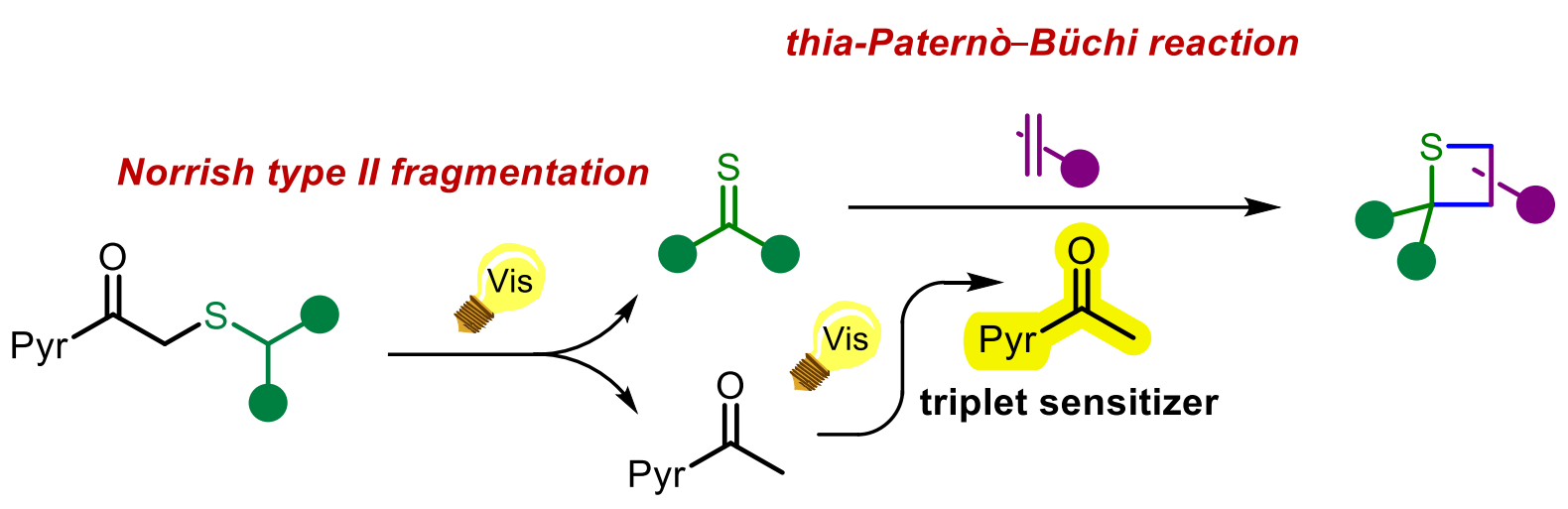
The reactions were performed under 405 nm light in a mixture of dichloromethane and ethyl acetate at either room temperature or –20 °C, using an excess of the alkene reaction partner. Under these conditions, the desired thietanes were obtained in mostly moderate to good yields. The work provides access to unprecedented thietane products. The team states that the photosensitization properties of 1-acetylpyrene could also be useful in other photochemical organic transformations.
- In Situ Generation of 1‐Acetylpyrene as a Visible‐Light Photocatalyst for the Thia‐Paternò‐Büchi Reaction,
Gabriel Cormier, Clémence Allain, Thomas Boddaert,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202412602