Evonik is researching how electrodialysis can be used on an industrial scale for green transformation in the chemical industry. Electrodialysis is a membrane-based process in which ions are transported across semi-permeable membranes using an applied electric field.
Electrodialysis can be used to separate salts that occur in many chemical processes and convert them back into their starting materials. Over the next five years, the company will invest a low double-digit million euro amount in the Electrochemical Processes & Products platform. Approximately 20 employees work in this area in Hanau, Germany, and Shanghai, China.
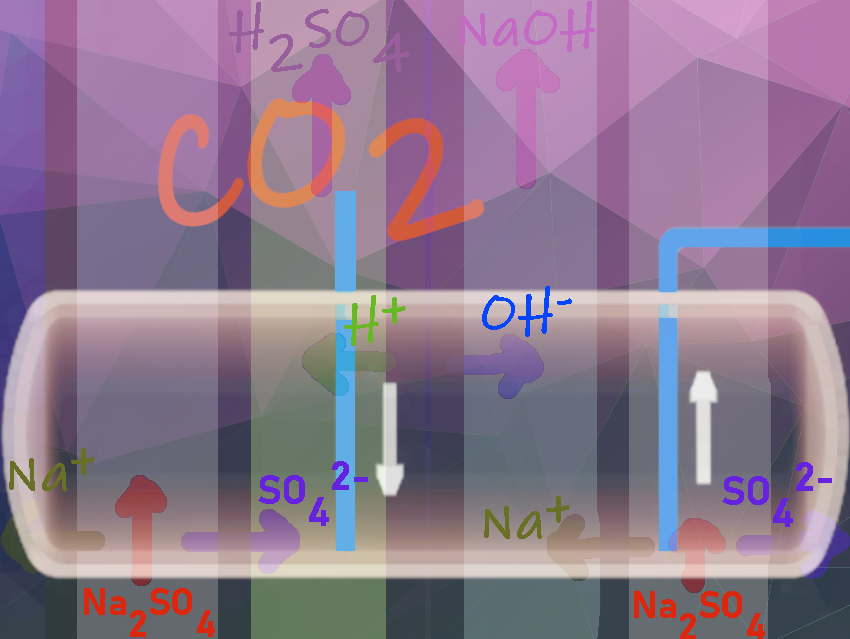
The company estimates that about 20 % of its processes alone can be made more efficient and environmentally friendly with the help of electrodialysis. This means lower energy consumption, less use of raw materials, lower CO2 emissions and less environmental pollution, for example from salt discharges. Depending on the price of raw materials and electricity, the recovery of raw materials from saline wastewater can pay for itself.
The challenge is to implement electrodialysis on a large scale. There are no off-the-shelf plants yet. The technology has to be adapted to the specific application and the substance to be separated. Another challenge is the membrane and its lifetime.
Examples Include
- pH value adjustment and recovery of raw materials: Using electrodialysis, salts produced during pH adjustment, such as sodium sulphate even from highly diluted solutions, can be converted into the raw materials such as caustic soda and sulphuric acid, which significantly reduces the CO2 footprint.
- Isophorone diamine production: The production of isophorone diamine, which is used in wind turbines, produces ammonium sulphate, from which ammonia and sulphuric acid could be recovered by electrodialysis – promising results have already been achieved.
- Evonik AG, Essen, Germany