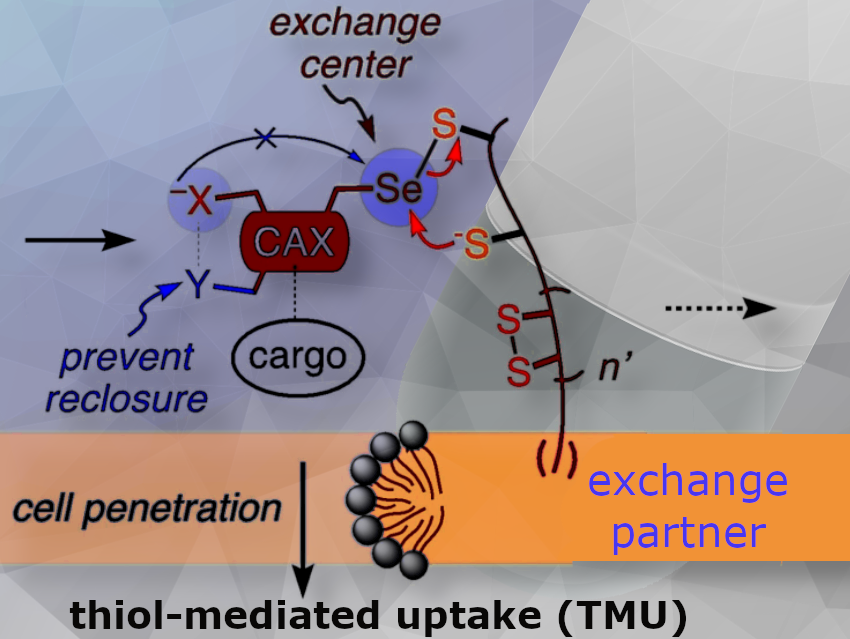
Thiol-mediated uptake (TMU) is a cellular entry mechanism into cells. It is based on the exchange of thiol groups (-SH) between disulfide bonds (-S-S-) present on the cell surface and thiol-containing molecules such as peptides, drugs, or nanoparticles. This process facilitates the transport of these molecules across the cell membrane by temporarily forming disulfide bonds with surface proteins, followed by reduction and release inside the cell, allowing for efficient intracellular delivery.
Stefan Matile, University of Geneva, Switzerland, Oliver Thorn-Seshold, Ludwig-Maximilians University of Munich, Germany, and co-workers introduce cyclic selenenylsulfides and remote exchange modulators to understand and control TMU in cells.
What did you do?
Thank you very much for your interest. We tested a new family of molecules for activation or inhibition of thiol-mediated uptake (TMU).
TMU is a long-known but poorly understood process. We hypothesized that TMU proceeds through a series of reversible reactions with biological thiols or disulfides, because the most active are cascade exchangers (CAXs), molecules that can engage in dynamic covalent exchange more than once.
The CAXs tested in this paper are cyclic selenenylsulfides with remote ammonium cations. Their ability to enable and inhibit TMU is explored in large screens against existing CAX collections.
Why is this of importance?
Cellular entry is so important for science and society, from drug delivery to drug discovery, and TMU emerges as a new way to do this. Although the power of TMU is increasingly recognized, nobody can say how it really works. We suspect this is so because the underlying dynamic covalent cascade exchange chemistry is so terribly difficult.
To decode the exchange networks at work, we need as many new CAXs as possible with unique properties. Unique properties should then generate unique patterns in TMU inhibitor screens that should reveal new networks with new cellular exchange partners.
What is new about your work?
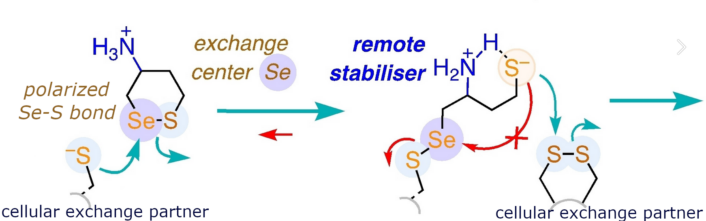
Our new CAXs promise the desired unique reactivity because, firstly, they operate with a polarized Se-S bond, never explored in TMU before.
Secondly, extra ammonium cations are added as remote exchange controllers. Depending on their position, they form ion pairs with anionic leaving groups and nucleophiles—here thiolates and selenolates—that appear and disappear during cascade exchange with disulfides and selenenylsulfides.
What are your key findings?
We find new TMU pathways and identify new cellular exchange partners. The consistency of the results is very satisfactory. This is perhaps the most important result. Rational design strategies afford the expected activities with high reliability. The concept of remote exchange controllers is particularly appealing because it is new and general. It could at best have a game-changing impact on TMU research, the future will tell.
Since ring-opening polymerization is closely related to cascade exchange in TMU, new CAXs designed to target the latter could also enable the development of modern dynamer materials that are both degradable and recyclable which is highly relevant to current needs.
Dynamer materials are a class of dynamic polymers formed by reversible covalent bonds that allow them to self-assemble, disassemble, and rearrange in response to external stimuli such as changes in temperature, pH, or the presence of certain chemicals. These materials show adaptive properties that allow them to heal, reconfigure, or change their physical properties dynamically, making them useful in areas such as drug delivery, self-healing materials, and responsive coatings.
What is the longer-term vision for your research?
If TMU is really confirmed to exist and matter as much as we suspect, it would be amazing to decode the underlying exchange networks completely. It could mean to achieve full control over cellular entry, deliver reliably whatever we want to deliver, and obstruct the entry of any pathogen we want to stop. Today, this looks like a big dream far beyond reach.
In the shorter term, we would like to develop the best CAXs into clickable traceless tags the community can use to solve daily delivery problems, and to strengthen the relation with dynamer materials. There seems to be so much room for really useful discoveries.
However, both objectives wont be met without understanding the underlying cascade exchange chemistry.
What part of your work was the most challenging?
We chemists all like the beauty and precision of molecular structure. However, in TMU, these well-defined structures become fuzzy as covalent bonds start to freely shift within and between molecules.
This is so demanding, no way to take pictures, use X-rays and beyond, no way to apply routine proteomics or molecular recognition methods. At the same time, dynamic covalent cascade exchange chemistry is so fascinating at the molecular level—not to speak of the cellular level. It’s wild and adventurous, and it will need supramolecular chemists to progress it.
Thank you very much for these insights.
The article they talked about
- Selenium-Centered Cascade Exchangers and Conformational Control Unlock Unique Patterns of Thiol-Mediated Cellular Uptake,
Filipe Coelho, Lukas Zeisel, Oliver Thorn-Seshold, Stefan Matile,
ChemistryEurope 2024.
https://doi.org/10.1002/ceur.202400032
Stefan Matile is a professor in the Department of Organic Chemistry at the University of Geneva, Switzerland.
Oliver Thorn-Seshold is a professor in the Department of Pharmacy at the Ludwig-Maximilians University of Munich, Germany.