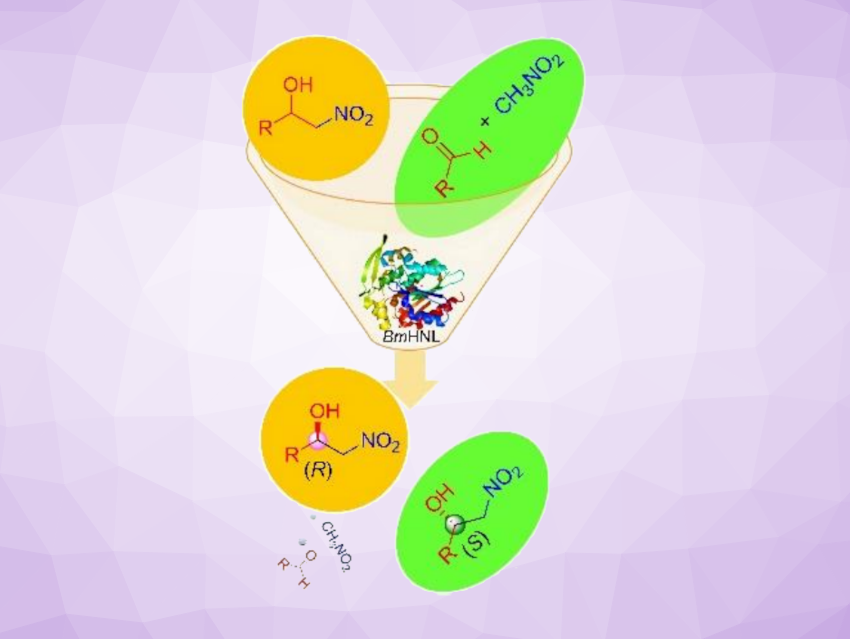
Enantioenriched β-nitroalcohols are versatile precursors to a number of pharmaceuticals and biologically active molecules. They are traditionally obtained using asymmetric Henry reactions using traditional catalysts. Biocatalysis can be a sustainable and effective alternative to existing chemical methods for the synthesis of chiral molecules.
Santosh Kumar Padhi, Central University, Hyderabad, India, and colleagues have developed a method for the biocatalytic synthesis of both (S)- and (R)-β-nitroalcohols using a single biocatalyst. Baliospermum montanum hydroxynitrile lyase (BmHNL), a cofactor-free enzyme, catalyzes both Henry and retro-Henry reactions, serving as a bidirectional catalyst to synthesize both (S)- and (R)-β-nitroalcohols (reactions pictured below).
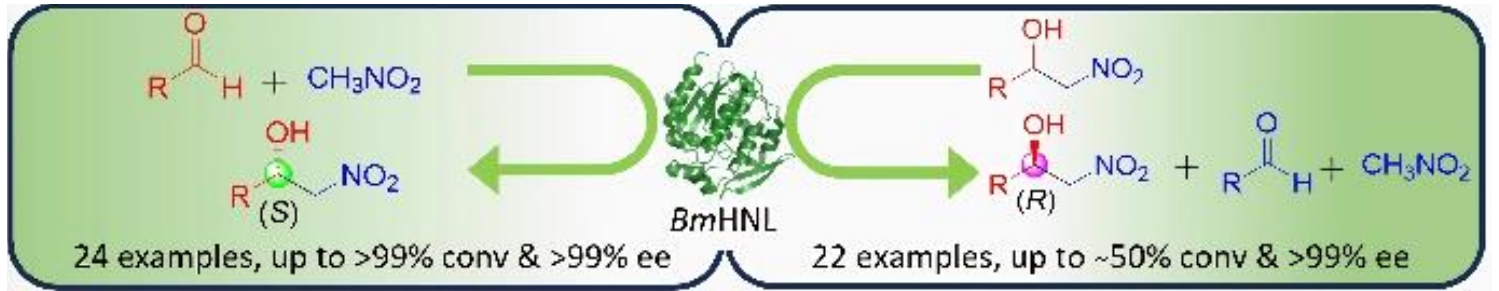
BmHNL showed broad scopes for both reactions. It was used for the synthesis of 46 chiral β-nitroalcohols with high enantioselectivity (up to > 99 % optical purity). The practical usability of this biocatalyst was demonstrated by the preparative-scale synthesis of chiral intermediates of drugs such as (S)-Tembamide and (S)-Micanozole on a millimolar scale. Overall, the researchers say their work provides a sustainable, scalable method for preparing chiral β-nitroalcohols.
- A Single Enzyme in Enantiocomplementary Synthesis of β‐Nitroalcohols: Bidirectional Catalysis by Hydroxynitrile Lyase,
Sukadev Rana, Ayon Chatterjee, Santosh Kumar Padhi,
ChemBioChem 2024.
https://doi.org/10.1002/cbic.202400618