Metallic lithium could be a useful anode material in batteries due to its ultrahigh theoretical capacity. However, metallic lithium anodes have significant drawbacks such as volume changes and the formation of lithium dendrites during charge/discharge cycles. This can cause electrolyte leakage or dangerous short circuits, possibly leading to fires or explosions. A suitable artificial solid electrolyte interface (SEI) layer, placed between the anode and the electrolyte, could prevent this.
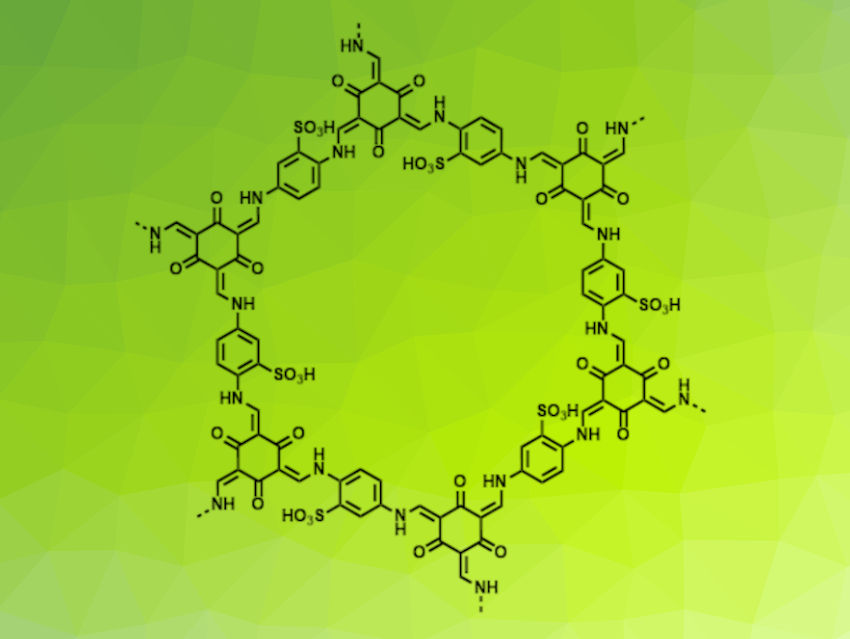
Yayuan Liu, Johns Hopkins University, Baltimore, MD, USA, and colleagues have developed a method for the preparation of a continuous, self-standing covalent organic framework (COF) membrane that can serve as an artificial SEI layer in lithium metal batteries. The team used the COF Tp-PaSO3H (structure pictured), which is synthesized from the monomers trihydroxybenzene-1,3,5-tricarbaldehyde (Tp) and 2,5-diaminobenzenesulfonic acid (PaSO3H). Usually, thin films of the COF are prepared at the interface between an organic phase containing Tp and an aqueous phase containing PaSO3H. However, this apporach generally gives fragile films and takes a long time.
The researchers have developed a same-phase synthesis method, in which both monomers are dissolved in n-octanoic acid as an organic phase (and activator for the aldehyde), and the base sodium acetate is present as an activator in the aqueous phase. A buffer layer of pure n-octanoic acid is added between these solutions, and the monomers have to diffuse through this layer to reach the interface. This leads to a slow activation of the PaSO3H monomer by the base at the interface and a controlled polymerization. The approach provides robust COF membranes that can be transferred onto other substrates and used as a protective layer on lithium metal anodes.
- A continuous covalent organic framework membrane as an artificial solid electrolyte interphase for lithium metal anodes,
Tae Jeong Kim, Xing Li, Fangzheng Chen, Yayuan Liu,
Chem. Commun. 2024.
https://doi.org/10.1039/D4CC02480J