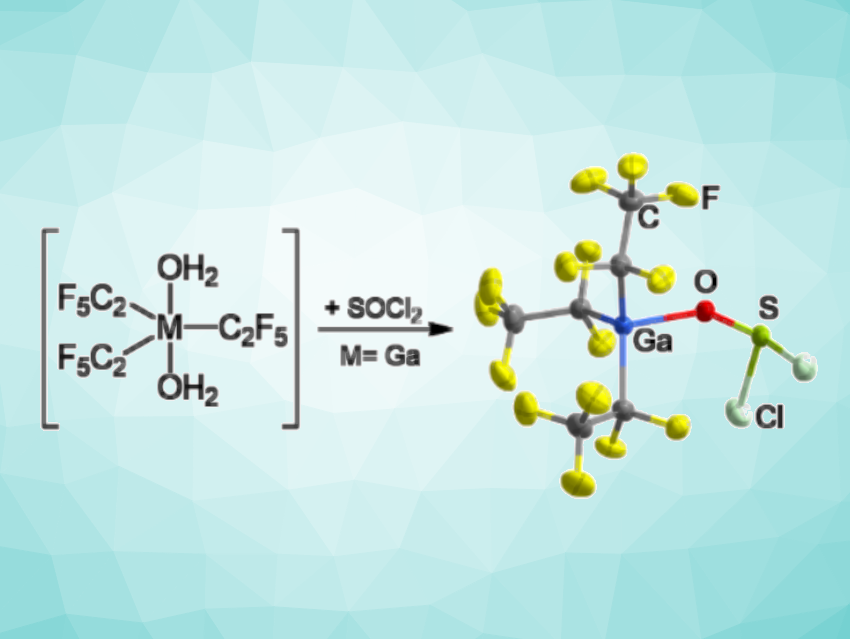
There are examples of strong Lewis acids based on main-group elements such as gallium and indium. While strong donor molecules can suppress the reactivity of strong Lewis acids, weak donors such as thionyl chloride (SOCl2) can lead to highly reactive complexes. Thus, such complexes can be challenging to synthesize and characterize. There are a few examples of transition-metal chloride adducts of thionyl chloride, but no structurally characterized adducts of thionyl chloride with Lewis acids based on main-group elements had been known so far.
Berthold Hoge, University of Bielefeld, Germany, and colleagues have synthesized tris(pentafluoroethyl)gallium and tris(pentafluoroethyl)indium adducts with weak donor molecules, including the first structurally characterized thionyl chloride adduct of a main group element Lewis acid, [Ga(C2F5)3(SOCl2)] (pictured). The team reacted tris(pentafluoroethyl)gallane or -indane dihydrate with thionyl chloride hoping to remove the water molecules. They found that these reactions created highly reactive products, and for the gallium substrate, they obtained crystals of [Ga(C2F5)3(SOCl2)] suitable for characterization by X-ray diffraction.
When the adducts formed from tris(pentafluoroethyl)gallane or -indane dihydrate and thionyl chloride were dissolved in an excess of fluorotrimethylsilane (Me3SiF), the thionyl chloride was substituted by Me3SiF and compounds of the type [M(C2F5)3(FSiMe3)] (M = Ga, In) were obtained. Both products were characterized using X-ray diffraction, and the team found that they form dimeric structures with contacts between one Ga or In atom and an F atom at the other monomer. Further complexes of Ga(C2F5)3 were obtained by substitution reactions. The researchers also investigated the catalytic activity of [M(C2F5)3(FSiMe3)] and found that the complexes can perform comparably to other Lewis superacids.
- The First Main-Group Element Lewis Acid Thionyl Chloride Adduct and Its Chemistry,
Katharina Tölke, Sven Porath, Beate Neumann, Hans-Georg Stammler, Berthold Hoge,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202408741