Cyclic peptides can be useful, for example, in pharmaceutical chemistry. While naturally occurring cyclic peptides have been used as a basis for drug development, methods for peptide cyclization are interesting research targets because they allow for the design of a wider range of peptide drug candidates. Linear peptide derivatives can be cyclized, e.g., using macrolactamizations, macrolactonizations, ring-closing metathesis reactions, the formation of disulfide bonds, or click reactions such as copper-catalyzed azide–alkyne cycloadditions (CuAAC) and thiol–ene reactions. The latter are reactions between a thiol and an alkene to form a thioether.
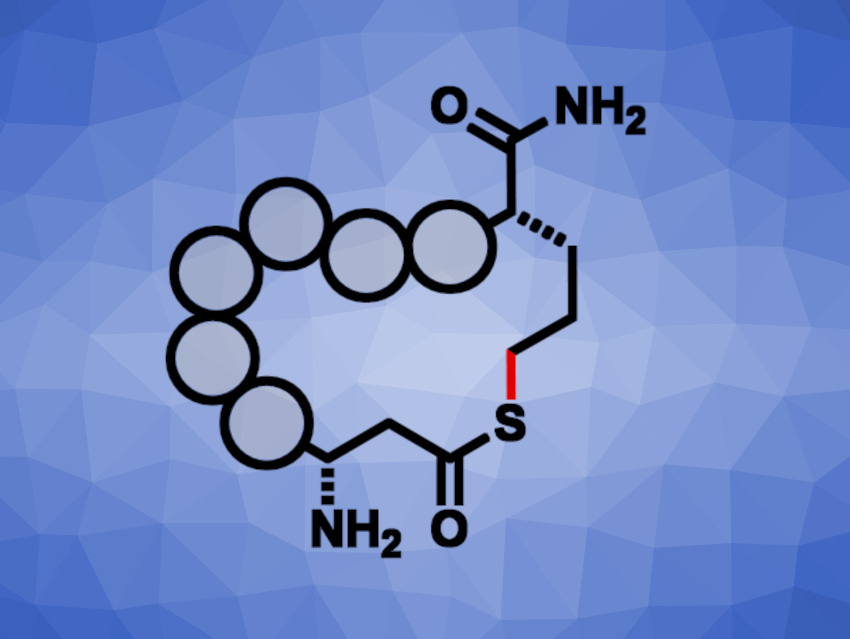
Eoin M. Scanlan, Trinity College Dublin, Ireland, and colleagues have developed a method for the rapid cyclization of peptide derivatives bearing a thioacid and an alkene group via a photochemical acyl thiol–ene reaction that forms a thiolactone (simplified product structure pictured). The team used 2,2-dimethoxy-2-phenylacetophenone (DPAP) as a photoinitiator and trifluoroacetic acid (TFA) as an additive. They performed the cyclizations under UV light irradiation in a mixture of water and acetonitrile at room temperature, with a reaction time of 15 min.
Under these conditions, the team cyclized different tetrameric or pentameric peptide derivatives that feature γ-thioaspartic acid and allylglycine residues. They obtained the desired macrocycles in moderate to good yields. According to the researchers, the work represents the first synthesis of peptide thiolactones under radical-mediated conditions. The speed of the reaction could make it useful for the generation of cyclic peptide libraries.
- Synthesis of macrocyclic thiolactone peptides via photochemical intramolecular radical acyl thiol–ene ligation,
Alby Benny, Eoin M. Scanlan,
Chem. Commun. 2024.
https://doi.org/10