Pharmaceutically active molecules that bind covalently to their targets have been used to develop successful therapies for different diseases. Covalent binding to target proteins is generally achieved using so-called electrophilic “warheads”, which are incorporated into the respective drug molecule and target nucleophilic amino acid residues. Acrylamides, for example, have been used as electrophilic groups in drugs used for the treatment of, e.g., lymphoma, leukemia, or lung cancer.
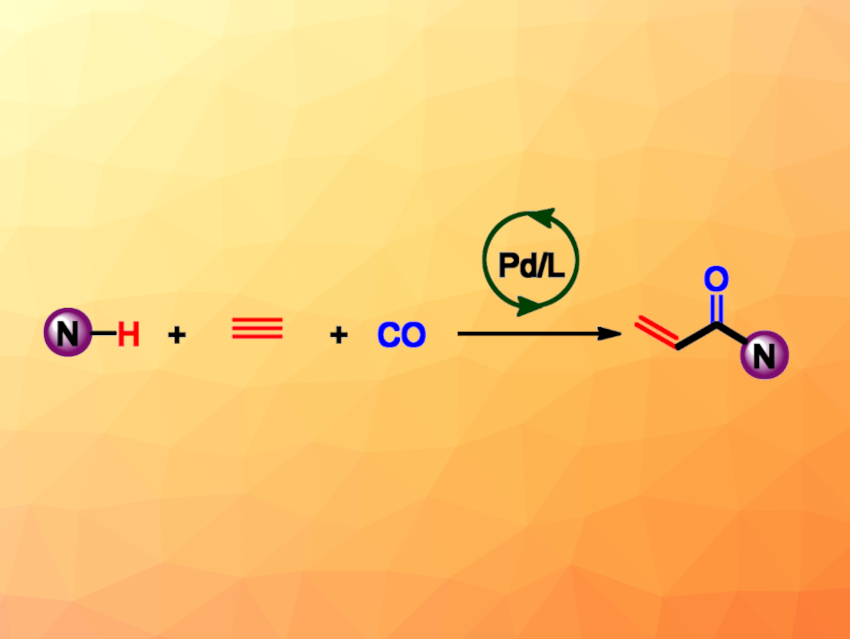
Helfried Neumann, Matthias Beller, Leibniz Institute for Catalysis. e.V., University of Rostock, Germany, and colleagues have developed a method for the modular synthesis of acrylamides via a palladium-catalyzed hydroaminocarbonylation of acetylene (general reaction pictured). The team used Pd(acac)2 (acac = acetylacetonate) as the catalyst together with a ferrocenylphosphine ligand to react a wide variety of amines with acetylene (1 bar) and CO (1 bar) in acetonitrile at 80 °C.
Under these conditions, the desired acrylamides were obtained in moderate to excellent yields. The researchers successfully converted a wide range of complex, drug-like amine substrates. The method showed broad functional group tolerance and good chemoselectivity. It has a high atom efficiency and can enable the straightforward synthesis of important drugs.
- Modular and Diverse Synthesis of Acrylamides by Palladium‐Catalyzed Hydroaminocarbonylation of Acetylene,
Matthias Beller, Zhusong Cao, Qiang Wang, Helfried Neumann,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202410597