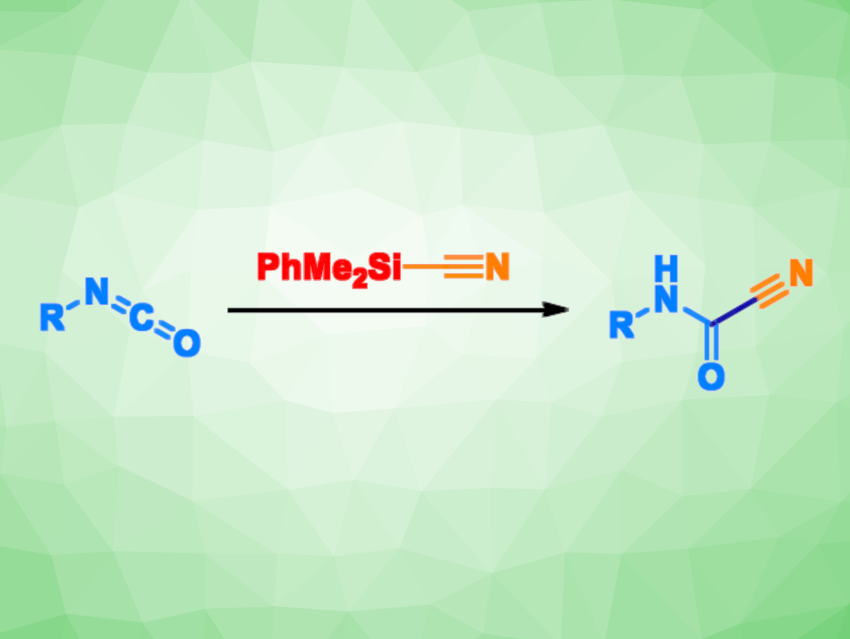
Cyanoformamides (RHN–C(=O)–CN) are useful intermediates in organic synthesis, e.g., for the preparation of heterocycles. However, their synthesis can require toxic CN-containing species and other hazardous reactants.
Vittorio Pace, University of Vienna, Austria, and University of Turin, Italy, and colleagues have developed a method for the straightforward preparation of cyanoformamides that is based on cyanide release from a suitable precursor in the presence of electrophilic isocyanates. The team used PhMe2SiCN as a cyanide “reservoir”, which releases CN– at a fast rate upon the formation and collapse of a complex with the Lewis base potassium tert-amylate (KOtAm).
The researchers reacted a variety of widely available isocyanates with different aromatic or aliphatic substituents with PhMe2SiCN in the presence of catalytic amounts of KOtAm in tetrahydronfuran (THF). The desired cyanoformamides were obtained within 1 min in high to excellent yields.
- Chemoselective Synthesis of Cyanoformamides from Isocyanates and a Highly Reactive Nitrile Anion Reservoir,
Margherita Miele, Laura Castoldi, Alexander Prado-Roller, Luisa Pisano, Vittorio Pace,
Eur. J. Org. Chem. 2024.
https://doi.org/10.1002/ejoc.202400619