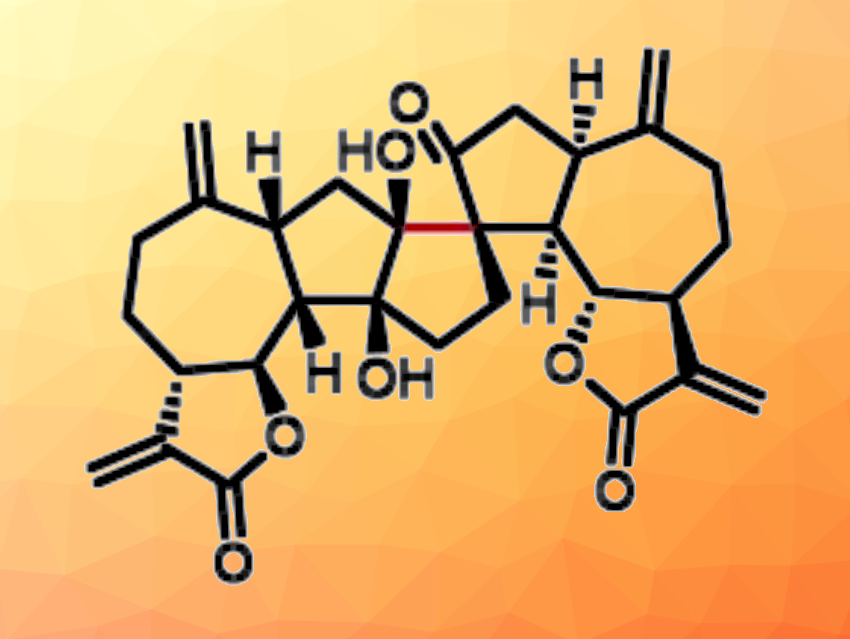
Sesquiterpenoid dimers are natural products with complex structures and interesting biological activities. For example, ainsliadimer A and gochnatiolide D, are compounds that were first isolated from natural plants and dimeric derivatives of dehydrozaluzanin C (compounds pictured below). They have promising anticancer/anti-inflammatory and cytotoxic activities, respectively. The compounds have complex structures and are challenging targets for total synthesis. Ainsliadimer A, for example, has a heptacyclic ring system with eleven contiguous stereocenters, including three quaternary carbon centers.
Alexandros L. Zografos, Aristotle University of Thessaloniki, Greece, and colleagues have developed a short one-pot total synthesis of gochnatiolide D and ainsliadimer A from their biosynthetic monomeric precursor dehydrozaluzanin C. This biomimetic procedure involves a highly diastereoselective Diels–Alder reaction of dehydrozaluzanin C, its subsequent hydrolysis to gochnatiolide D, and the spontaneous aldol reaction of gochnatiolide D to ainsliadimer A. The process (pictured below) was enabled by hexafluoroisopropanol (HFIP), which was used as the solvent.
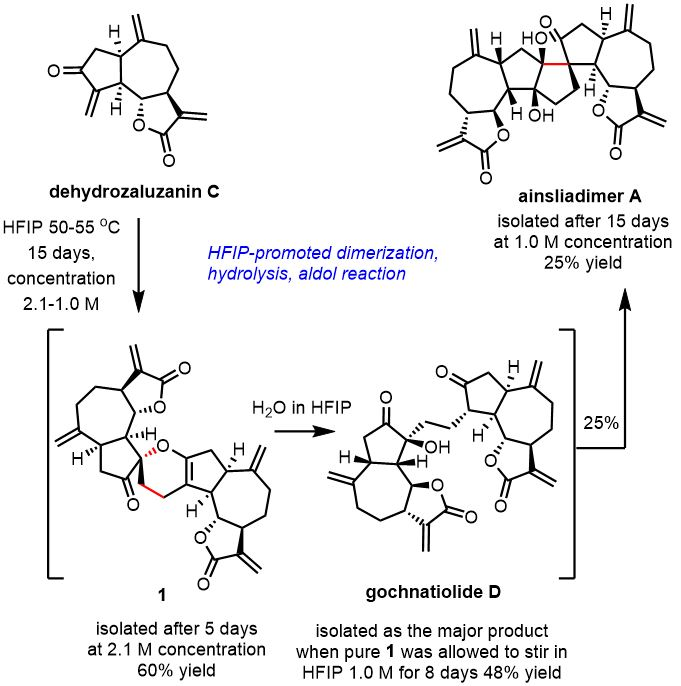
The hydrogen-bond donor properties of HFIP help to initiate the process of dimerization via a Diels–Alder reaction. Then, the acidic character of HFIP promotes the hydrolysis of intermediate 1 to give gochnatiolide D. Finally, the hydrogen-bond donating ability of HFIP stabilizes an enol intermediate, leading to the final product via an aldol reaction. According to the researchers, the work is an example of reaction conditions mimicking the biogenetic environment leading to the highly stereoselective synthesis of complex natural products under operationally simple conditions.
- HFIP‐Promoted Short Total Synthesis of Gochnatiolide D and Ainsliadimer A from Dehydrozaluzanin C,
Antonis Kelesidis, Kyriaki Gennaiou, Alexandros L. Zografos,
Eur. J. Org. Chem. 2024.
https://doi.org/10.1002/ejoc.202400533