Organic reactions are often catalyzed using rare and expensive transition metals. Replacing these metals with more Earth-abundant ones, such as iron, could make the reactions more sustainable and cost-effective. For example, radical hydrofunctionalizations of alkenes can be synthesized using manganese, iron, or cobalt-based catalysts.
Christos I. Stathakis, Aristotle University of Thessaloniki, Greece, and colleagues have investigated cobalt-catalyzed Mukaiyama hydration reactions of allylic alcohols and observed an unexpected reaction: In addition to the hydration product, a Cα–Cβ cleavage product was formed. Switching the catalyst from cobalt to iron (Fe(acac)3, acac = acetylacetonate) led to the exclusive formation of the cleavage product.
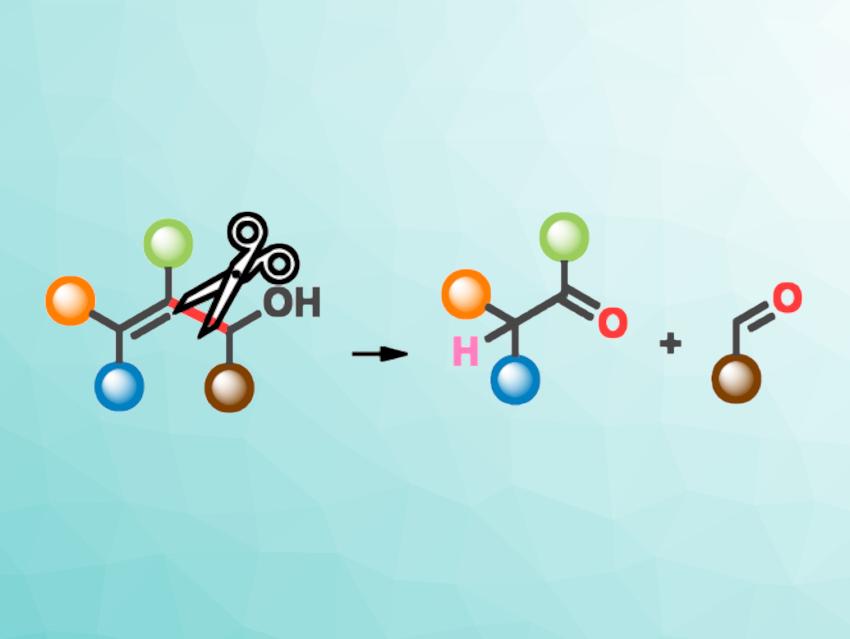
The team optimized the reaction conditions for this transformation and converted a variety of allylic alcohols to the corresponding ketones and aldehydes (general reaction pictured)—or ring-opened products in the case of cyclic allylic alcohols. They used Fe(acac)3 as the catalyst in the presence of PhSiH3 and O2 as the oxygen source. The reactions were performed in ethanol at 25 °C.
The desired products were obtained in moderate to high yields. They can be further functionalized, and the reaction could be useful in the construction of complex molecular structures. The transformation has a wide substrate scope and proceeds under mild aerobic conditions.
- Iron(ΙΙΙ) Catalyzed Aerobic Cα–Cβ Cleavage of Allylic Alcohols,
Christos I. Stathakis, Georgia G. Bagkavou,
Eur. J. Org. Chem. 2024.
https://doi.org/10.1002/ejoc.202400556