Halogenation reactions can be important in living organisms to, e.g., change the bioactivity of molecules. Vanadium-dependent haloperoxidases are a family of enzymes that promote halogenations. They use a vanadate cofactor, hydrogen peroxide, and halide ions. Some of these enzymes can promote site-selective halogenations, but they have a small scope of known substrates.
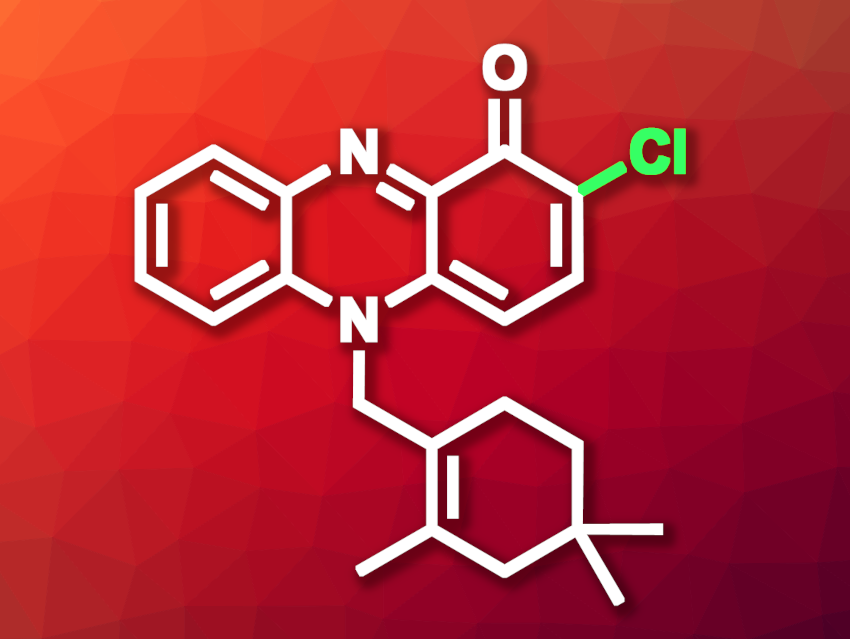
Shaun M. K. McKinnie, University of California, Santa Cruz, USA, and colleagues have searched for new substrates of vanadium-dependent haloperoxidases, using Streptomyces bacteria. The bacteria produce halogenated natural products, such as halogenated phenazines (dibenzo-annulated pyrazines). Derivatives of the phenazine-derived meroterpenoid lavanducyanin, for example, show interesting bioactivities, but their full biosynthetic route remains unknown (C2-chlorinated derivative, or azulocyanin, pictured).
The team extracted lavanducyanin, azulocyanin, and the brominated derivative marinocyanin A from a Streptomyces strain cultured in artificial seawater, indicating that halogenation reactions took place. They performed a genomic analysis of the bacteria, which pointed to the involvement of vanadium-dependent haloperoxidases.
The researchers found a new regioselective vanadium-dependent haloperoxidase, lavanducyanin halogenase (LvcH). According to the team, LvcH is the first reported phenazinone halogenase. It was used in chemoenzymatic synthesis and might be useful in the development of new halogenation biocatalysts.
- Regioselective Halogenation of Lavanducyanin by a Site-Selective Vanadium-Dependent Chloroperoxidase,
Jackson T. Baumgartner, Shaun M. K. McKinnie,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c01869