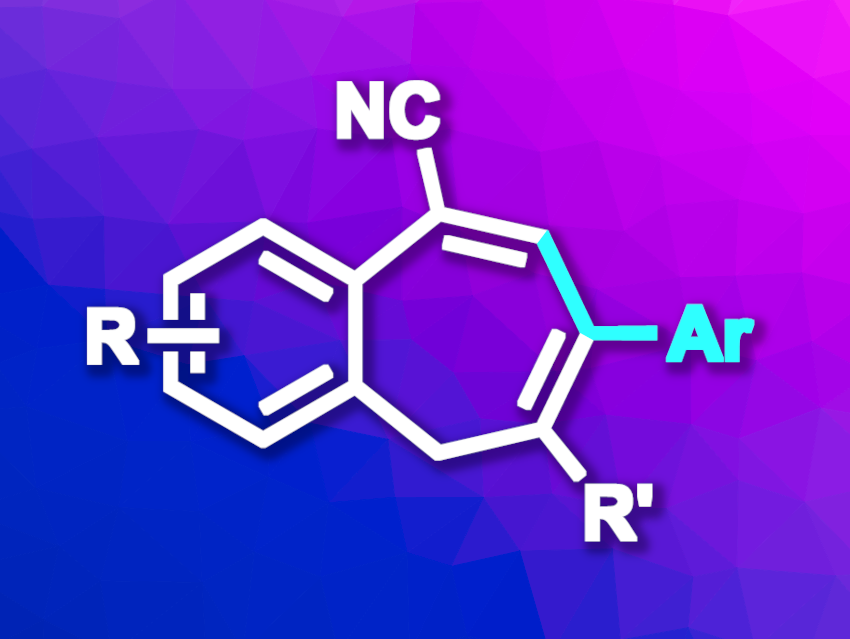
Unsaturated seven-membered rings fused to a benzene unit can be found, e.g., in some natural products and bioactive compounds. Methods for their synthesis can have drawbacks such as a narrow range of substrates or a need for harsh reaction conditions. (5+2) Cyclizations can be practical paths to seven-membered rings, but there is a lack of stable, easy-to-obtain C5 building blocks for this type of reaction.
Wanqing Wu, South China University of Technology, Guangzhou, China, and colleagues have developed a method for the synthesis of benzocycloheptene derivatives that uses 2-(alkynylaryl)acetonitriles as C5 building blocks (general product structure pictured). The approach involves a base-promoted (5+2) annulation of 2-(alkynylaryl)acetonitriles and arylalkynes. The team reacted a variety of 2-alkynylarylacetonitriles with a range of different aryl-substituted primary alkynes using t-BuOK as a base, LiN(SiMe3)2 as an additive, and tetrahydrofuran (THF) as the solvent. The reactions were performed at 90 °C.
Under these conditions, the desired benzocycloheptenes were obtained in moderate to high yields. The reaction was successfully performed on a gram scale. The products can be further transformed using, for example, their CN group. The method is transition-metal-free, has a broad substrate scope as well as good atom economy, and proceeds under mild reaction conditions.
- Base-Promoted (5 + 2) Annulation between 2-(Alkynylaryl)acetonitriles and Arylalkynes for the Synthesis of Benzocycloheptene Derivatives,
Jiatian Li, Xiangwen Tan, Huanfeng Jiang, Wanqing Wu,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c00940