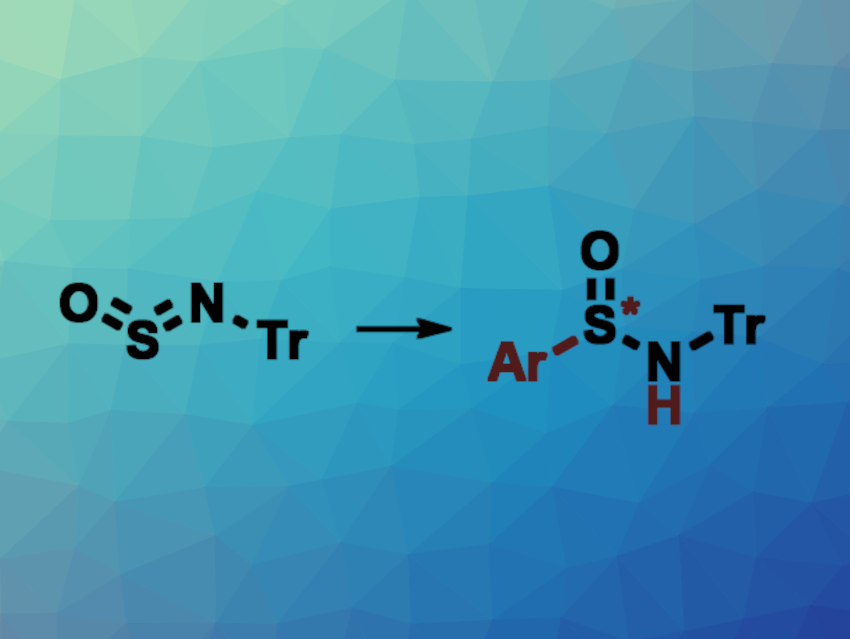
Sulfinylamines (R–N=S=O) can serve as useful building blocks in organic synthesis. They can, for example, be converted to sulfinamides (R2N−S(=O)–R). One approach to this is the nickel-catalyzed addition of (hetero)aryl boroxines to N-sulfinyltritylamine (Tr–N=S=O, pictured above on the left, Tr = trityl). Asymmetric methods for producing chiral sulfinamides from sulfinylamines are rare, but would be useful.
Minyan Wang, Nanjing University, China, Zhuangzhi Shi, Nanjing University, Nanjing Normal University, China, and Henan Normal University, China, and colleagues have developed a method for the asymmetric 2,3-addition of arylboronic acids to sulfinylamines using nickel catalysis to ultimately give chiral sulfinamides (general overall reaction pictured). The team used NiCl2(dme) (dme = dimethoxyethane) as a nickel catalyst together with a chiral bisoxazoline ligand, Cs2CO3 as a base, and 1,4-dioxane as the solvent in the presence of 4 Å molecular sieves to react a variety of arylboronic acids with N-sulfinyltritylamine.
Under these conditions, the desired chiral sulfinamides were obtained in moderate to excellent yields and with high enantioselectivities. The products are stable and can be further transformed into other useful S-stereogenic derivatives.
- Asymmetric 2,3-Addition of Sulfinylamines with Arylboronic Acids Enabled by Nickel Catalysis,
Longlong Xi, Xiaowu Fang, Minyan Wang, Zhuangzhi Shi,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c04050