Drugs that are administered in an inactive, “caged” form and then activated at specific places and times using, for example, light can be useful, e.g., in cancer treatment. This can have advantages such as as fewer or less severe side effects or a lower likelihood for the development of resistances.
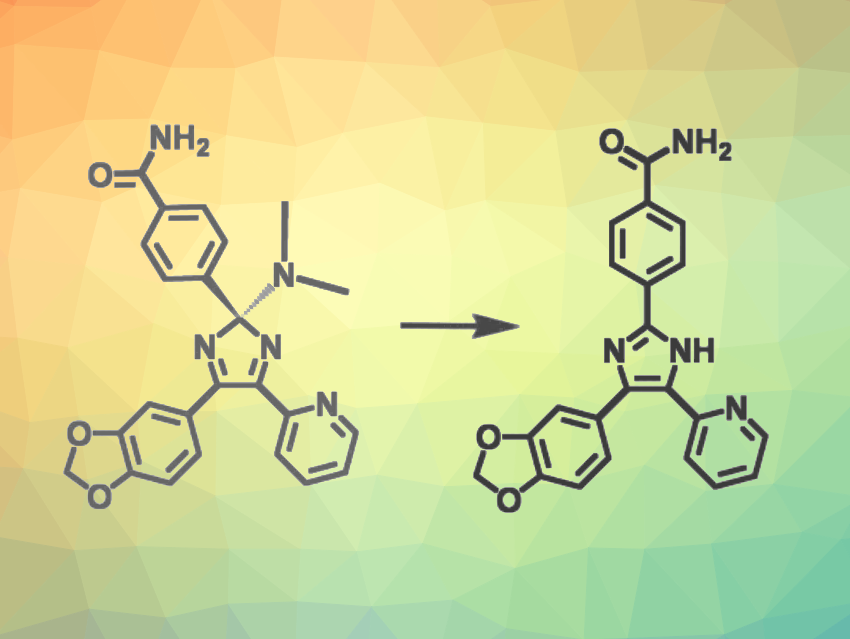
Nobuyuki Tamaoki, Hokkaido University, Japan, and colleagues have developed a new strategy for caging bioactive triarylimidazoles (example pictured), using a dialkylamino unit as a photoremovable group. The team focused on the compound SB431542 (pictured above on the right), a triarylimidazole-based small-molecule inhibitor that targets the tumor-promoting actions of TGF-β type 1 receptors in human cancer cells and inhibits colony formation by certain late-stage colon cancer cells. They introduced a dimethylamino group at the carbon atom of the imidazole ring via a ferrocyanide oxidation in the presence of dimethylamine in N,N-dimethylformamide (DMF).
The dialkylamino group changes the core structure from a planar imidazole to a tetrahedral 2H-imidazole and inactivates the drug, which can be selectively uncaged upon visible-light exposure. The researchers found that blue light in the 400–450 nm range is suitable for activating the drug by cleaving the bond to the dialkylamino group. The team performed assays using human breast cancer cells and found that the approach could be used to selectively target cancer cells within specific regions without harming healthy cells.
- Caging Bioactive Triarylimidazoles: An Approach to Create Visible Light-Activatable Drugs,
Jiajun Qi, Ammathnadu S. Amrutha, Sumire Ishida-Ishihara, Hisham M. Dokainish, P. K. Hashim, Ryu Miyazaki, Masumi Tsuda, Shinya Tanaka, Nobuyuki Tamaoki,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c04468