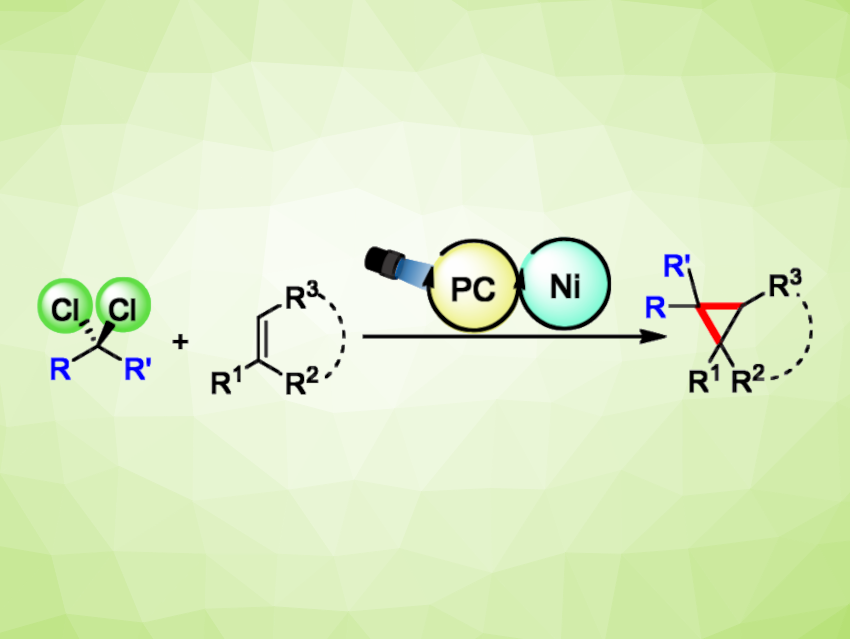
Cyclopropanes are strained three-membered rings that are often found, e.g., in biologically active compounds. They can serve as bioisosteres (i.e., chemically similar groups that produce similar biological properties) of 1,2-disubstituted benzene rings. Still, introducing cyclopropane units during the chemical synthesis of, e.g., drug candidates, can be challenging. In particular, efficient cyclopropanation methods that use a commonly available C1 synthon (building block) without a need for stoichiometric quantities of toxic or difficult-to-handle reagents would be useful.
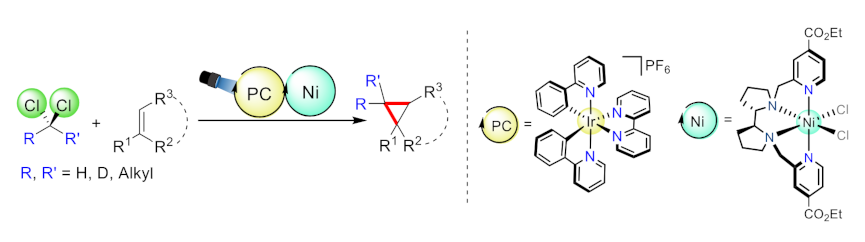
Julio Lloret-Fillol, Institut Català d’Investigació Química (ICIQ), Barcelona Institute of Science and Technology (BIST), Tarragona, Spain, and Institució Catalana de Recerca i Estudis Avançats (ICREA), Barcelona, Spain, and colleagues have developed a method for the cyclopropanation alkenes with aromatic substituents using low-cost dichloromethane as a C1 building block (general reaction pictured). The team used a dual photoredox system composed of a nickel aminopyridine complex and an iridium photocatalyst (pictured below on the right).
The reactions were performed in acetonitrile at 30 °C under blue LED light (447 nm) in the presence of N,N-diisopropylethylamine (DIPEA). The desired cyclopropane derivatives were obtained in mostly high yields. The reaction was successfully performed at the gram scale, giving a yield of 82 %. The method showed a broad functional group tolerance, working well with a variety of different functional groups at the aromatic substituent as well as a range of heteroaromatic substituents or polycyclic aromatic groups. Trisubstituted cycloalkenes were also well-tolerated. Other geminal dichloroalkanes can be used instead of dichloromethane. When the reaction is performed using CD2Cl2, deuterium-labelled cyclopropanes are obtained.
- Dichloromethane as C1 Synthon for the Photoredox Catalytic Cyclopropanation of Aromatic Olefins,
Jordi Aragón, Suyun Sun, Sergio Fernández, Julio Lloret-Fillol,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202405580