Molecules with multiple bonds involving the heavier main group elements can show interesting reactivities, for example, in the activation of small molecules. In particular, examples with double bonds between different elements could show increased reactivity due to the higher polarity of the bond in question. In this context, phosphagermenes, i.e., compounds with P=Ge double bonds and their potential for small-molecule activation are not well explored because they can be difficult to synthesize.
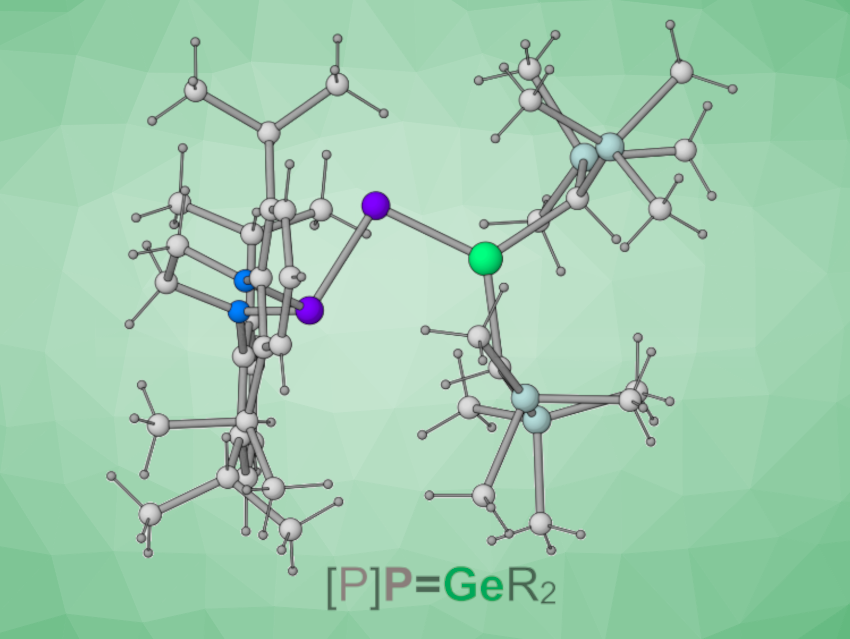
Jose M. Goicoechea, Indiana University, Bloomington, USA, and colleagues have synthesized the phosphanyl phosphagermene [{(H2C)(NDipp)}2P]P=Ge[CH(SiMe3)2]2 (pictured above, Dipp = 2,6-diisopropylphenyl) and evaluated its reactivity toward small molecules. The team synthesized the target compound by reacting the phosphaketene [{(H2C)(NDipp)}2P]P=C=O with the germylene Ge[CH(SiMe3)2]2. The desired product was formed under release of CO in a yield of 58 %, and its structure was confirmed using single crystal X-ray diffraction.
The team then looked at the reactivity of the phosphanyl phosphagermene toward small molecules such as H2, CO2, and NH3. They observed no reaction with H2. With CO2 (1 bar at 60 °C), the compound undergoes a [3+2]-cycloaddition to give a five-membered heterocycle (pictured below). This type of product is known from reactions of frustrated Lewis pairs (FLPs) with CO2. The nitrile MeCN reacts analogously. The team proposes that the phosphanyl phosphagermene reacts like a geminal FLP with these molecules, with the phosphanyl group acting as the base and the germanium center as the Lewis acid.

With NH3, as well as small primary amines (NH2R’) or metal complexes such as Au(PPh3)Cl, the phosphanyl phosphagermene undergoes 1,2-addition reactions across the P=Ge bond (example pictured above). Thus, the new compound shows two distinct reactivity modes.
- A Phosphanyl Phosphagermene and its Reactivity,
Joey Feld, Eric Yang, Stephanie Urwin, Jose Goicoechea,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202401736