Metal hydride complexes play important roles in some catalytic processes. They tend to form polynuclear structures due to intermolecular metal–hydride interactions. Most known polynuclear metal hydride clusters have three-dimensional polyhedral structures.
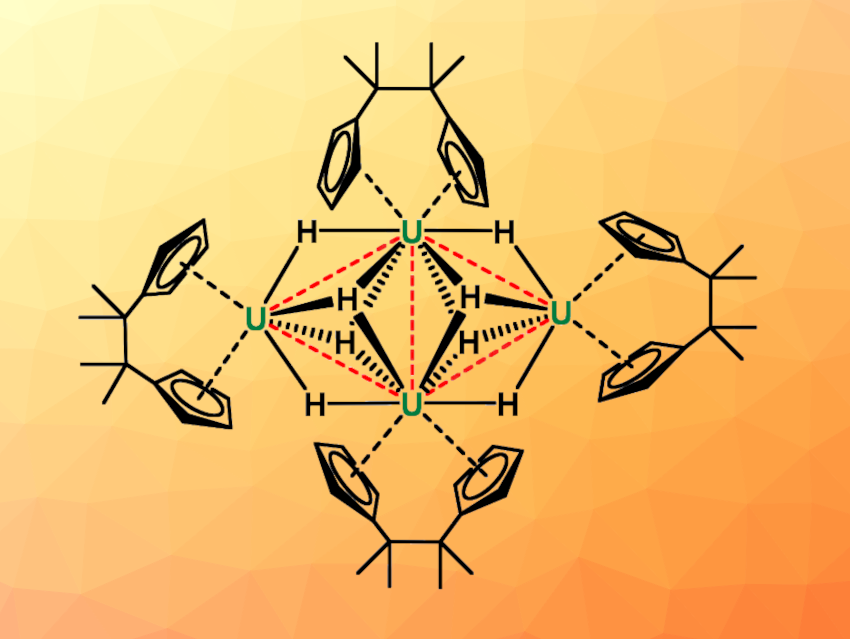
Laurent Maron, Université Paul Sabatier, Toulouse, France, Congqing Zhu, Nanjing University, China, and colleagues have synthesized the first example of a planar tetranuclear uranium hydride cluster, namely, [(CpCMe2CMe2Cp)U]4(μ2-H)4(μ3-H)4 (Cp = cyclopentadienyl). The team first synthesized a uranium-chloride-based precursor with the desired ansa-bis(cyclopentadienyl) ligand system, [(CpCMe2CMe2Cp)U]3(μ2-Cl)3(μ3-Cl)2, from UCl4 and dimethylpentafulvene in the presence of magnesium. This precursor was then reacted with NaBHEt3 in tetrahydrofuran (THF) at room temperature for 10 h to obtain the tetranuclear uranium hydride cluster.
The product was characterized using X-ray single-crystal analysis, and the team found that the four uranium atoms form a planar rhombic arrangement, bridged by eight hydrides (pictured above). Each uranium atom is coordinated to one ansa-bis(cyclopentadienyl) ligand. According to the researchers, the cluster is both the first planar polynuclear actinide metal hydride cluster and the uranium hydride cluster with the highest nuclearity reported so far.
Planar Tetranuclear Uranium Hydride Cluster Supported by ansa‐Bis(cyclopentadienyl) Ligands,
Kai Li, Iker del Rosal, Yue Zhao, Laurent Maron, Congqing Zhu,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202405494