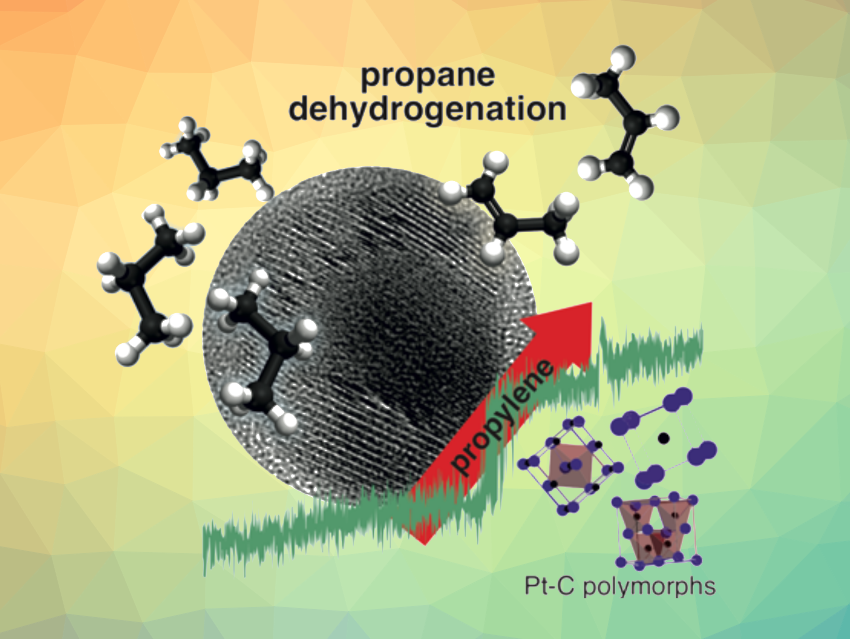
The dehydrogenation of propane to produce propylene, catalyzed, e.g., by Pt nanoparticles, is an important reaction in the chemical industry. Propylene is used for the production of a variety of chemicals, including polypropylene and propylene oxide. So far, little is known about the solid-state chemistry that occurs during this transformation at the Pt catalysts and about the structures that form under the working conditions. A better understanding of these questions could help to improve propylene production.
Thomas Götsch and Thomas Lunkenbein, Fritz Haber Institute of the Max Planck Society, Berlin, Germany, and colleagues have studied the structural and chemical changes in Pt nanoparticle catalysts during propane dehydrogenation. The team used operando transmission electron microscopy (TEM), selected area electron diffraction (SAED), and mass spectrometry (MS), as well as density functional theory (DFT) calculations to evaluate how the catalyst changes during the reaction and how the changes influence propylene production.
Using MS measurements, the researchers monitored the conversion of propane to propylene while raising the reaction temperature. They found that propylene production increases sharply at temperatures between 438 °C and 474 °C. This jump correlates with the appearance of ordered Pt-C solid solutions, in particular, a CsCl-type Pt-C polymorph. At higher temperatures, a ZnS-type polymorph or a mixture of a NaCl-type Pt-C polymorph and the ZnS-type Pt-C polymorph dominated the observed structures, and the propylene production was not enhanced as significantly. Overall, the work contributes to the understanding of what structures can contribute to an active and selective catalyst for propane dehydrogenation.
- In situ Formation of Platinum‐Carbon Catalysts in Propane Dehydrogenation,
Hannah C. Nerl, Milivoj Plodinec, Thomas Götsch, Katarzyna Skorupska, Robert Schlögl, Travis E. Jones, Thomas Lunkenbein,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202319887