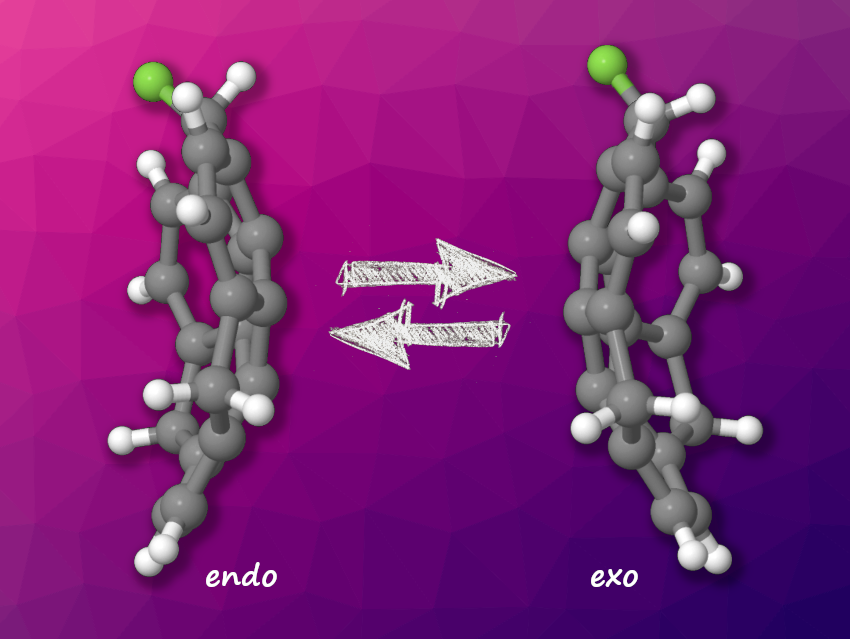
“Buckybowls” are bowl-shaped molecules that resemble parts of fullerenes such as C60. They can undergo bowl-to-bowl inversion, in which they “flip” between two configurations. This could be useful, e.g., in molecular switches that change upon an external signal. Sumanene is a buckybowl that consists of a central benzene unit surrounded by alternating benzene and cyclopentadiene units. The benzylic carbon atoms on the five-membered rings can be functionalized, and a substituent in this position can either point “into” the bowl (endo conformation) or to the “outside” of the bowl (exo configuration). A bowl inversion causes a switch between these two configurations.
Yumi Yakiyama, Osaka University, Japan, and colleagues have studied monofluorosumanene, which has a single F substituent. The team synthesized the compound from hydroxysumanene, which was reacted with diethylaminosulfur trifluoride (DAST) in CH2Cl2 to give the fluorinated derivative in a yield of 43 %. They used density functional theory calculations to evaluate the stability of the endo and exo conformations of the molecule (pictured above) and found that the endo form is more stable. In solution, this was confirmed using NMR spectroscopy. The endo/exo ratio depends on the solvent and the temperature.
The researchers then prepared crystals of monofluorosumanene using different solvents for crystallization. They found that the endo/exo in the resulting crystals was significantly different for different solvents. When CH2Cl2 was used, the endo and exo configurations appeared in similar amounts in the crystals, while for dimethylformamide (DMF), the exo configuration was more common (71–82%, depending on the temperature). These differences also cause a change in properties, in particular, different dielectric responses of the crystals. This insight could open up new uses of this bowl-inversion effect for the preparation of materials.
- Biased Bowl-Direction of Monofluorosumanene in the Solid State,
Yumi Yakiyama, Minghong Li, Dongyi Zhou, Tsuyoshi Abe, Chisato Sato, Kohei Sambe, Tomoyuki Akutagawa, Teppei Matsumura, Nobuyuki Matubayasi, Hidehiro Sakurai,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c11311