3H-Indole N-oxides and their analogs, found in natural products and bioactive molecules, show diverse biological properties, including antifungal and antibacterial properties. Despite existing synthetic methods, there’s a continued need for more efficient approaches to construct diverse 3H-indole N-oxides, particularly for synthesizing C7-functionalized isatogens (indolin-3-one N-oxides).
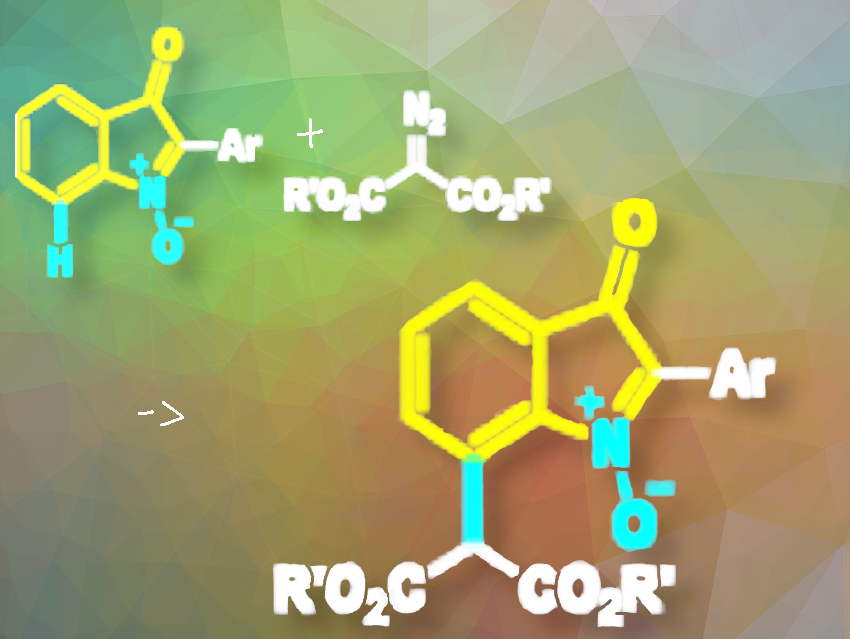
Yong-Long Zhao and colleagues, Guizhou Medical University, Guiyang, P.R. China, have developed a Rh(III)-catalyzed C7-alkylation of isatogens (indolin-3-one N-oxides) with malonic acid diazoesters as the environmentally benign alkyl source (pictured). The team performed the reaction with [Cp*RhCl2]2 (transition-metal catalyst), CsOAc (base), AcOH, TBAB (tetrabutylammonium bromide; phase transfer reagent), and toluene (solvent), under a N2 (balloon) atmosphere at 130 °C.
Their method uses the oxygen anion on the N-oxide group of isatogens (pictured in yellow) as a directing group for the C7–H bond cleavage, enabling the synthesis of a range of C7-alkylated isatogens with moderate to good yields (48–86% yields). The C7-alkylation reaction showed good tolerance to various functional groups of isatogens.
The researchers propose a reaction mechanism involving the formation of an active [CpRh(III)] metal species ([Cp*Rh(OAc)2]) from [CpRhCl2]2 in the presence of CsOAc. This species facilitates the formation of a five-membered rhodacycle intermediate via N-oxide-directed C7-H bond cleavage of the isatogen. The intermediate then coordinates with the malonic acid diazo ester to form a second intermediate, which releases N2 to generate a six-membered rhodacycle third intermediate. The process is not entirely clear. Finally, the six-membered rhodacycle intermediate is protonated to yield the desired C7-alkylation product.
- Rh(III)-Catalyzed C7-Alkylation of Isatogens with Malonic Acid Diazoesters,
Xiang Guan, Wen-Jie Li, Ming-Shan Shuai, Mao Zhang, Chao-Chao Zhou, Xiao-Zhong Fu, Yuan-Yong Yang, Meng Zhou, Bin He, Yong-Long Zhao,
J. Org. Chem. 2024.
https://doi.org/10.1021/acs.joc.3c02405