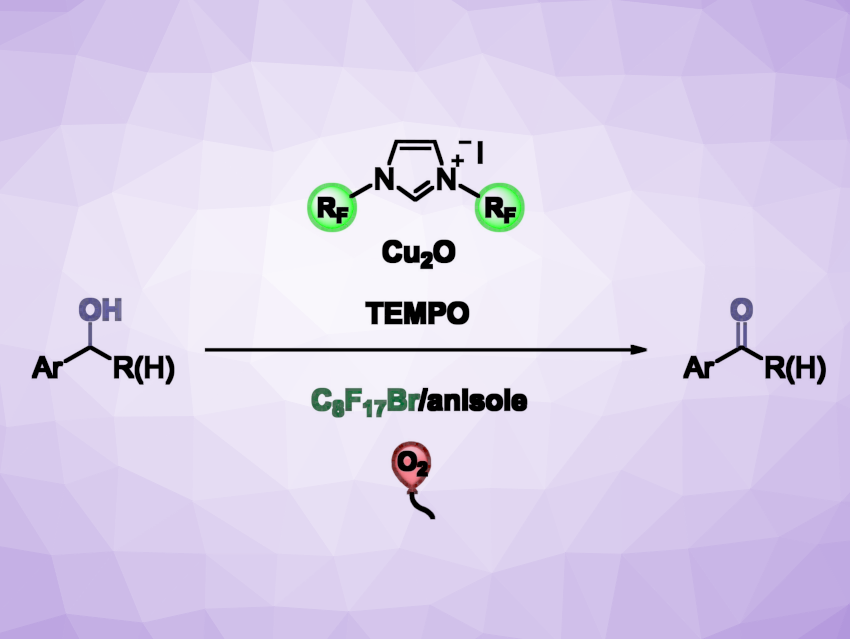
Transition-metal complexes in which N-heterocyclic carbenes (NHCs) with perfluorinated substituents serve as ligands can show remarkable solubility in fluorous (i.e., highly fluorinated) solvents. This can be used to create biphasic catalytic systems, enabling the effective recycling of the catalysts and the straightforward separation and purification of the products.
Georgios C. Vougioukalakis, National and Kapodistrian University of Athens, Greece, and colleagues have developed a fluorous biphasic catalytic protocol for the aerobic oxidation of benzyl alcohols. The approach uses an in situ-generated catalytic system consisting of a widely available and cost-efficient copper source (Cu2O), easily accessible NHC ligand precursors with perfluorinated substituents, and TEMPO (2,2,6,6-tetramethyl-piperidyl-1-oxyl), together with oxygen as the oxidant. The transformation takes place at the interface of a biphasic solvent system, consisting of anisole and 1-bromoperfluorooctane.
This method allows the facile separation of the catalytic system from the product by decantation of the organic phase. The researchers found that the catalyst is efficient even at low loadings. The protocol works well for primary alcohols, affording the desired products in moderate to excellent yields, and it tolerates a variety of functional groups. The fluorous phase containing the catalytic system can be reused at least six times without a significant decrease in catalytic activity.
- An In‐Situ‐Formed Copper‐Based Perfluorinated Catalytic System for the Aerobic Oxidation of Alcohols,
Maria Drymona, Entzy Kaplanai, Georgios C. Vougioukalakis,
Eur. J. Org. Chem. 2024.
https://doi.org/10.1002/ejoc.202301179