Saturated nitrogen-containing heterocycles are often found, e.g., in natural products, pharmaceutically active compounds, or agrochemicals. The selective functionalization of the C–H bonds of simple cyclic amines can be useful for the synthesis of more complex azaheterocyclic compounds. There is a range of available methods for the 2-functionalization of such amines, while the functionalization of other C–H bonds—or multiple C–H bonds—in these cyclic systems can be more challenging.
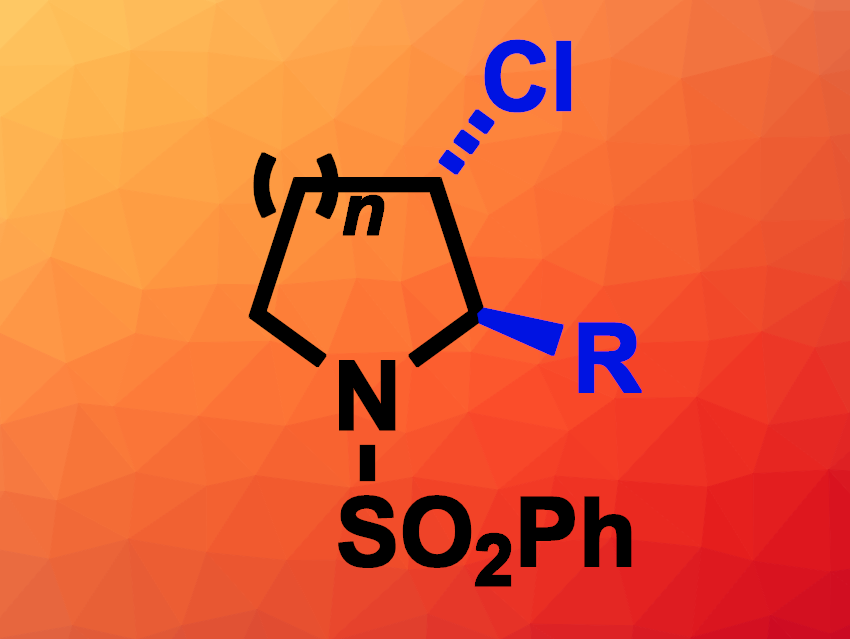
Tristan H. Lambert, Cornell University, Ithaca, NY, USA, and colleagues have developed an electrochemical method for the functionalization of two vicinal (neighboring) C–H bonds of saturated azaheterocycles (general product structure pictured). The team reacted a variety of different phenylsulfonyl-protected pyrrolidines and piperidines (as well as an azepane) with a mixture of hydrochloric acid, acetic acid, and acetic anhydride in an undivided electrochemical cell at a constant current, using graphite (pencil lead) as the anode, a Pt wire as the cathode, tetraethylammonium tetrafluoroborate as the electrolyte, and CH2Cl2 as the solvent. This reaction gives α-acetoxy-β-chloro derivatives.
The resulting α-acetoxy-β-chloro intermediate can then undergo substitution reactions in the α-position to introduce a range of other groups, including alkyl, aryl, allyl, alkynyl, alkoxy, or azido groups. In addition, the β-chloro substituent can be used for Suzuki cross-coupling reactions. Thus, the approach allows for the easy diversification of five-, six-, and seven-membered saturated azaheterocycles in two neighboring positions.
- Electrochemical Vicinal C–H Difunctionalization of Saturated Azaheterocycles,
Gourab Kundu, Tristan H. Lambert,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c12336


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
