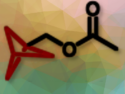
Dendralenes are acyclic cross-conjugated polyenes that can be useful, e.g., in the synthesis of natural compounds or polymers. [3]Dendralenes are the simplest cross-conjugated dendralenes, featuring three double bonds. They can be used, e.g., in Diels–Alder reactions to build complex structures.
Lijuan Song, Harbin Institute of Technology, Shenzhen, China, Yun-Dong Wu, Shenzhen Bay Laboratory, China, and Peking University Shenzhen Graduate School, China, Jianwei Sun, The Hong Kong University of Science and Technology (HKUST), Hong Kong SAR, China, and Shenzhen Research Institute, China, and colleagues have developed a method for the synthesis of densely substituted [3]dendralenes via the ruthenium-catalyzed intermolecular dimerization of allenes (general product structure pictured). The approach uses unactivated 1,1-disubstituted allenes without prefunctionalization at the allylic position as substrates.
The team used [Cp*RuCl2]n as the catalyst and dimethylformamide (DMF), dichloromethane (DCM), or chlorobenzene (PhCl) as the solvent. The reactions were performed at room temperature under a nitrogen atmosphere. The desired [3]dendralenes were obtained in moderate to excellent yields. The method tolerates a variety of functional groups and provides good to high E/Z stereocontrol.
- Mild Stereoselective Synthesis of Densely Substituted [3]Dendralenes via Ru-Catalyzed Intermolecular Dimerization of 1,1-Disubstituted Allenes,
Shijia Li, Qiang Feng, Lijuan Song, Xinhao Zhang, Yun-Dong Wu, Jianwei Sun,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c11448
![Dimerization of Allenes Gives [3]Dendralenes](https://www.chemistryviews.org/wp-content/uploads/2024/01/3dendralenesviadimerizationofallenes_2024.png)