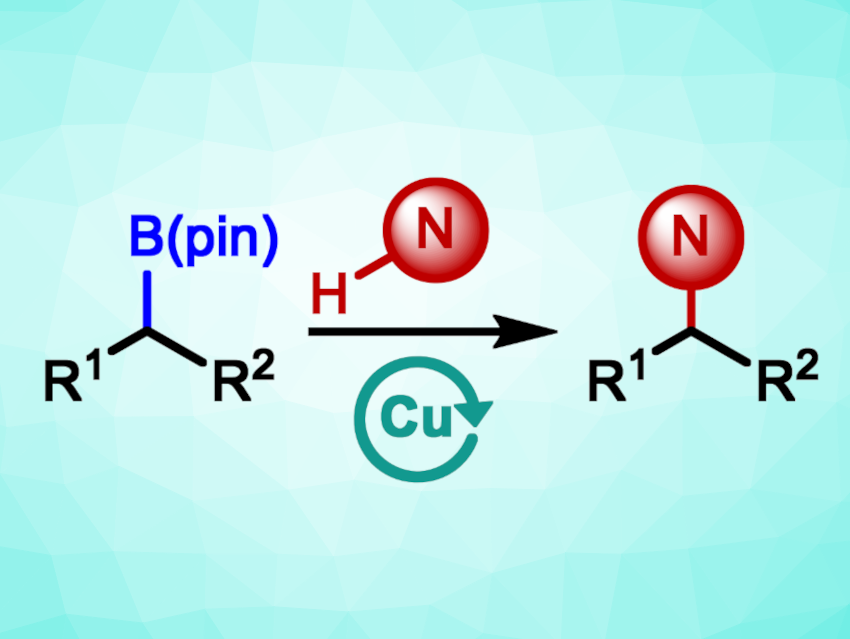
Alkyl amines are widely used in applications from medicinal chemistry to materials science. There are reliable methods to prepare aryl amines, which are routinely used. The preparation of alkyl amines can be somewhat more challenging. Traditional methods used to synthesize alkyl amines can suffer from poor selectivity and a need for protecting groups.
Benjamin M. Partridge, University of Sheffield, UK, and colleagues have developed a method for the copper-catalyzed oxidative coupling of aliphatic amines with benzylic and aliphatic boronic esters to give alkyl amines (pictured schematically). The team reacted a variety of boronic esters with a broad range of cyclic and acyclic secondary amines, as well as primary aliphatic amines, using CuBr2 as the catalyst, a mixture of toluene and isopropanol as the solvent, and O2 from air as the terminal oxidant. The reactions were performed at 80 °C.
The desired products were obtained in moderate to high yields. The reaction tolerates a broad range of functional groups, and it was successfully used as the final step in the synthesis of a complex drug-like compound. Based on mechanistic studies, the researchers suggest that the reaction proceeds via an alkyl radical intermediate that is generated from the boronic ester upon activation by an aminyl radical.
- Cu‐Catalyzed Coupling of Aliphatic Amines with Alkylboronic Esters,
Francesca Dennis, Antonio Romero Arenas, George Rodgers, Muralidharan Shanmugam, Jonathan Andrews, Samantha Peralta-Arriaga, Benjamin Michael Partridge,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202303636