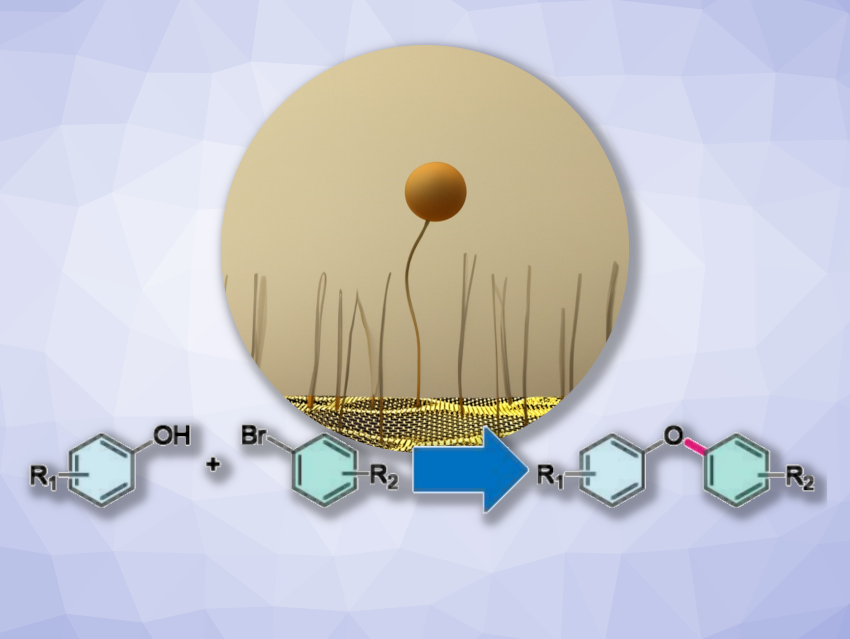
Cross-coupling reactions play a crucial role in the construction of complex organic molecules. One example is the Ullmann-type C–O coupling, which involves combining phenols and aryl halides to give diaryl ethers. Usually, homogeneous catalysts are employed for this, but they can have drawbacks such as low stability and difficulty in recovering and reusing the catalyst. Heterogeneous systems in which metals are anchored on solid supports could be a useful alternative.
Gianvito Vilé, Politecnico di Milano, Italy, and colleagues have developed a new type of catalyst for Ullmann-type C−O coupling reactions. The catalyst consists of single copper atoms bonded to a tetraethylenepentamine-pyrrole ligand, which is anchored to graphite nanoplatelets. The team first prepared the ligand via a Paal-Knorr reaction of the polyamine N1-(2-aminoethyl)-N2-(2-((2-aminoethyl)amino)ethyl)ethane-1,2-diamine with 2,5-hexanedione. The ligand was reacted with graphene nanoplatelets at 180 °C, undergoing a Diels–Alder reaction which binds it to the graphene support. Finally, a copper salt is added to form the desired catalyst.
The catalyst provides high activity, selectivity, and recyclability. The catalyst preparation process is environmentally friendly, and the system avoids the use of external ligands, leading to easier purification and isolation of the reaction products. Overall, the work extends the potential of single-atom catalyst nanoarchitectures and provides a customized catalyst for cross-coupling reactions.
- Copper Single Atoms Chelated on Ligand‐Modified Carbon for Ullmann‐type C–O Coupling,
Vincenzo Ruta, Giovanni Di Liberto, Francesco Moriggi, Yurii P. Ivanov, Giorgio Divitini, Gianlorenzo Bussetti, Vincenzina Barbera, Mark A. Bajada, Maurizio Galimberti, Gianfranco Pacchioni, Gianvito Vilé,
ChemSusChem 2023.
https://doi.org/10.1002/cssc.202301529