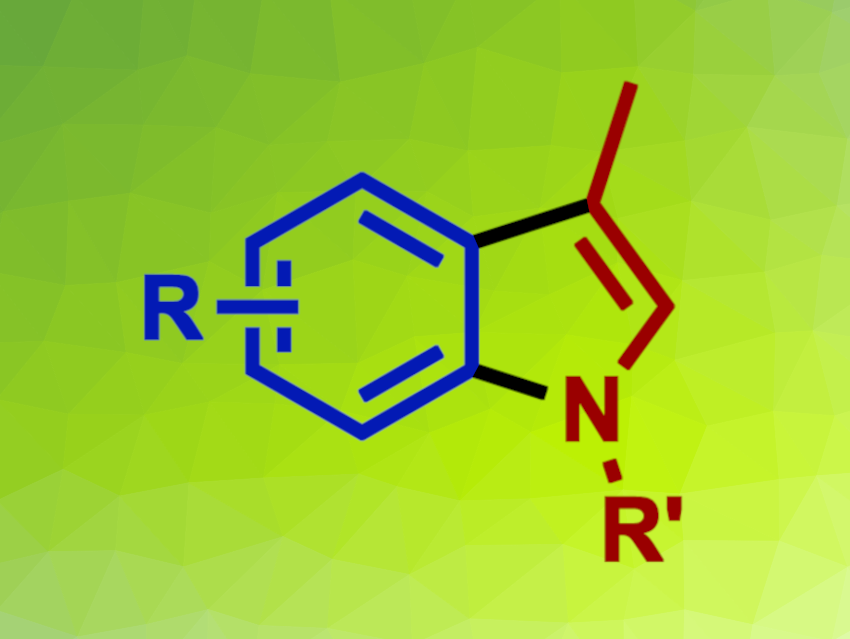
Indoles are important heterocycles. 3-Methylindoles, in particular, can be useful, e.g., in pharmaceutical chemistry. Methods that provide straightforward synthetic access to 3-methylindoles are, thus, interesting research targets.
Yuanzhi Xia, Wenzhou University, China, Weiwei Fang, Nanjing Forestry University, China, and colleagues have developed a catalytic strategy for the synthesis of N-substituted 3-methylindoles from ortho-dihaloarenes and N-allylamines via a cyclization reaction. The team used an oxazoline palladacycle with a 1,3-bis(2,6-diisopropylphenyl)acenaphthoimidazol-2-ylidene (AnIPr) ligand as the catalyst, together with tBuONa as a base. The reactions were performed at 100 °C in dioxane.
Using this approach, the desired 3-methylindole products were obtained in mostly good to excellent yields. The protocol has a broad substrate scope. The team proposes a mechanism that involves an amination, followed by a Heck reaction. According to the researchers, the method can be used for the synthesis of several bioactive compounds and key intermediates in the preparation of natural products.
- A General Protocol toward Synthesis of 3-Methylindoles Using Acenaphthoimidazolyidene-Ligated Oxazoline Palladacycle,
Ruoqian Fan, Haili Wen, Zhen Chen, Yuanzhi Xia, Weiwei Fang,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c03438