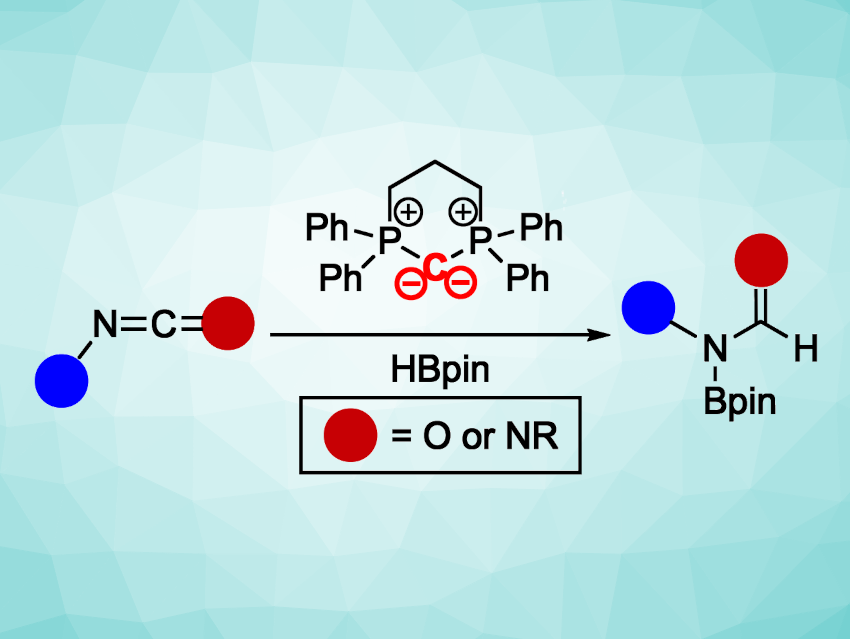
Reducing carbodiimides (RN=C=NR) and isocyanates (RN=C=O) is an appealing route to formamidine and formamide functional groups, which appear, for example, in biologically active compounds. However, the presence of multiple polar double bonds in these heterocumulene-type substrates can make selective reduction reactions challenging to achieve.
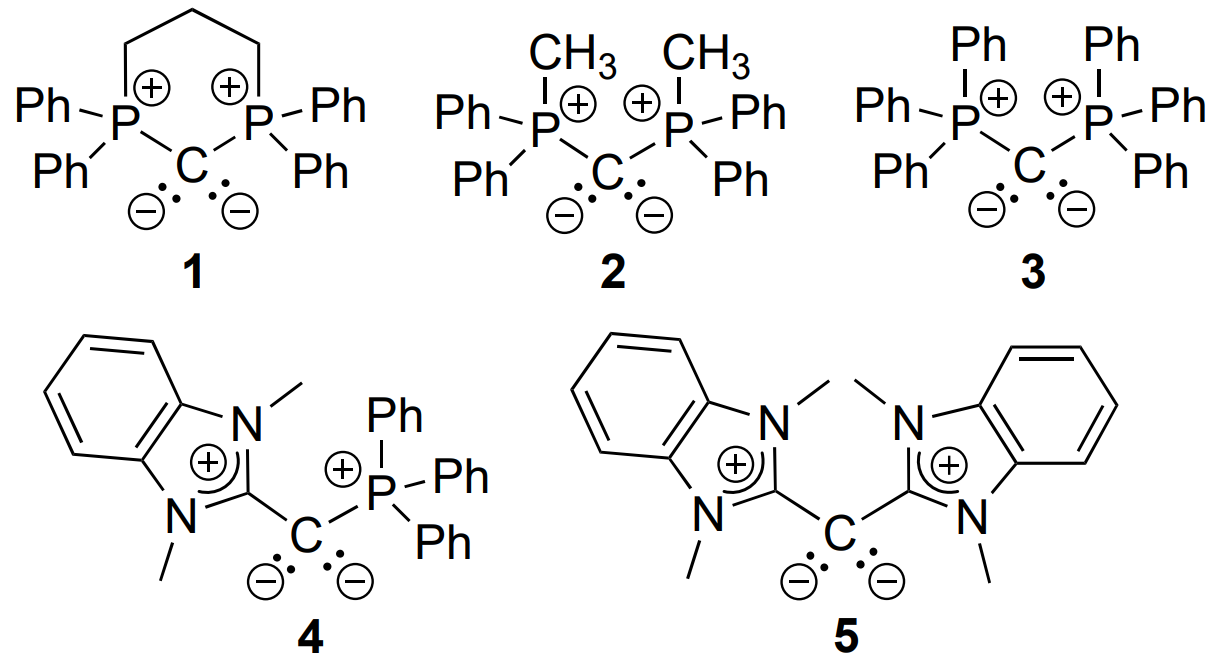
O. Maduka Ogba, Allegra L. Liberman-Martin, Chapman University, Orange, CA, USA, and colleagues have developed a method for the selective hydroboration of carbodiimide and isocyanate substrates (pictured above) using Lewis-basic carbon catalysts. The team compared different classes of neutral carbon Lewis-basic catalysts, including both cyclic and acyclic carbodiphosphoranes (1–3), a carbophosphinocarbene (4), and a carbodicarbene (5). The cyclic carbodiphosphorane 1 showed the best catalytic activity.
Using this catalyst, the team performed hydroboration reactions of seven carbodiimides and nine isocyanates with pinacolborane at room temperature. The desired N-boryl formamidine and N-boryl formamide products were obtained in yields up to 99 %. Computational studies indicate a likely mechanism involving catalyst coordination to the pinacolborane, followed by a hydride transfer and B–N bond formation. According to the team, the work represents the first report of carbodiimide or isocyanate reduction catalyzed by a neutral, Lewis-basic organocatalyst.
- Carbodiimide and Isocyanate Hydroboration by a Cyclic Carbodiphosphorane Catalyst,
Ben A. Janda, Julie A. Tran, Daniel K. Chang, Gabriela C. Nerhood, O. Maduka Ogba, Allegra L. Liberman-Martin,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202303095