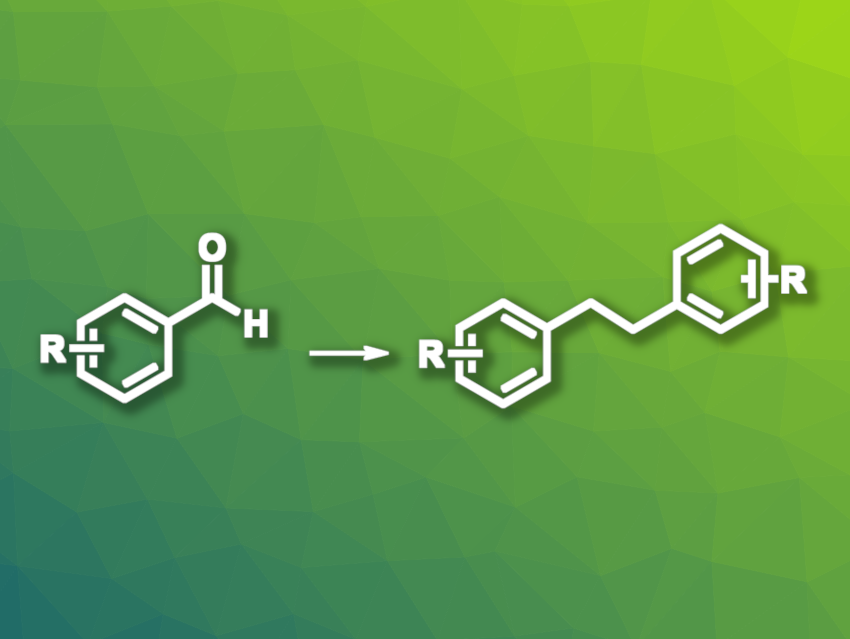
Bibenzyl-based structures (i.e., 1,2-diphenylethane derivatives) can be found, e.g., in pharmaceutically active compounds or dyes. They can be obtained, for example, via coupling reactions such as the reductive homo-coupling of benzyl halides. This type of reaction often requires transition-metal catalysts or photocatalysts, as well as harsh reaction conditions. Milder and more sustainable approaches would be useful. Aromatic aldehydes could be promising alternative starting materials for the synthesis of bibenzyls.
Lifang Tian, Yahui Wang, Nanjing Tech University, China, and colleagues have developed a method for the electrochemical deoxygenative homo-coupling of aromatic aldehydes to give bibenzyl and stilbene derivatives. The team transformed a variety of aromatic aldehydes in an undivided electrochemical cell with a graphite anode and a nickel cathode at a constant current of 20 mA, using acetonitrile as the solvent and Et4NBr as the electrolyte in the presence of triphenylphosphine and a catalytic amount of acetic acid.
The desired bibenzyl derivatives were obtained in moderate to good yields. The protocol can be modified, using dichloromethane (DCM) as the solvent, to give stilbene products instead, albeit with low yields in some cases. The team proposes a mechanism that involves a single-electron-reduction of the aldehyde to give the corresponding radical anion, followed by homo-coupling to give a diol-based intermediate. The intermediate reacts with a triphenylphosphine cation radical and can then give a stilbene derivative with the release of two stable triphenylphosphine oxides, or be further oxidized and undergo a pinacol-type rearrangement to form carbonyl compounds. These intermediates can then again react with a triphenylphosphine cation radical and form bibenzyls.
- Electrochemical deoxygenative homo-couplings of aromatic aldehydes,
Xiaoqian Zhao, Meng Li, Kunhui Sun, Zhimin Xu, Lifang Tian, Yahui Wang,
Chem. Commun. 2023.
https://doi.org/10.1039/D3CC03346E