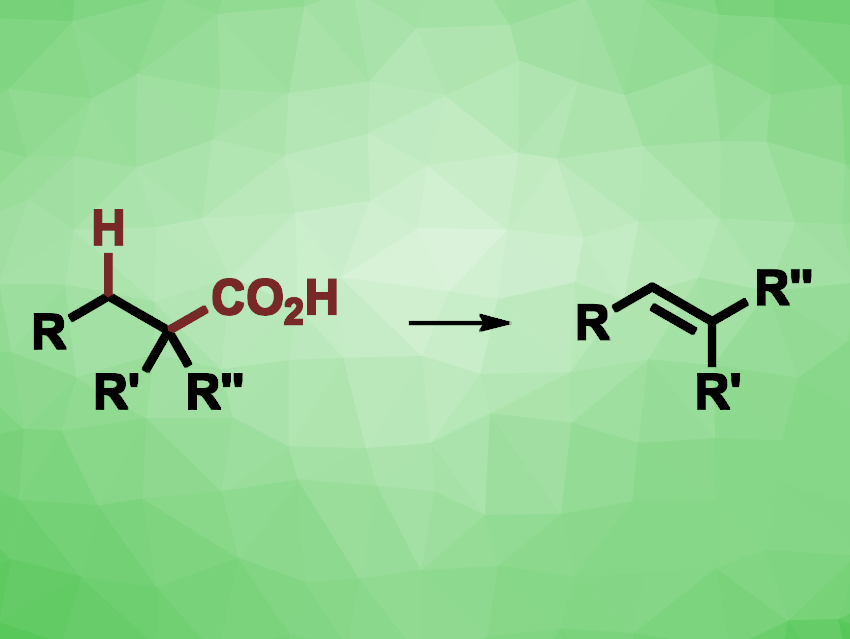
Alkenes are valuable products and intermediates in organic synthesis, and new methods for their synthesis are always interesting research targets. Elimination reactions starting from alcohols or alkyl halides are common, but tend to require excess reagents. The direct decarboxylative elimination of readily available carboxylic acids could be an environmentally friendly alternative approach.
Yunfei Zhang, China Agricultural University, Beijing, China, and colleagues have developed an electrochemical strategy for the decarboxylative elimination of carboxylic acids to alkenes under mild conditions. The team used an undivided electrochemical cell with carbon felt as both anode and cathode, using nBu4NOAc as the electrolyte and acetonitrile as the solvent. Different 2,2-diaryl propionic acids and α-aryl aliphatic carboxylic acids were subjected to electrolysis at room temperature.
The team obtained the desired alkene products in moderate to good yields. The electrochemical reaction proceeded smoothly on a gram scale, providing 56 % yield in a test reaction. The team proposes a mechanism that involves the deprotonation of the carboxylic acid, followed by electrooxidation to generate a carboxylate radical. CO2 is then released, giving a carbon-centered radical. Oxidation and deprotonation steps then give the desired alkene. At the cathode, hydrogen gas is released. According to the researchers, the method is attractive due to the use of abundant carboxylic acid feedstocks, the mild, oxidant-free reaction conditions, and its scalability.
- Electrochemical Decarboxylative Elimination of Carboxylic Acids to Alkenes,
Jiage Yu, Teng Liu, Wanhao Sun, Yunfei Zhang,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c02997