Microbes can upgrade renewable chemical feedstocks via multi-step transformations with built-in co-factor regeneration, delivering value-added products under controlled, green conditions. However, enzyme selectivity and toxicity limit the scope of products accessible via biosynthetic means alone.
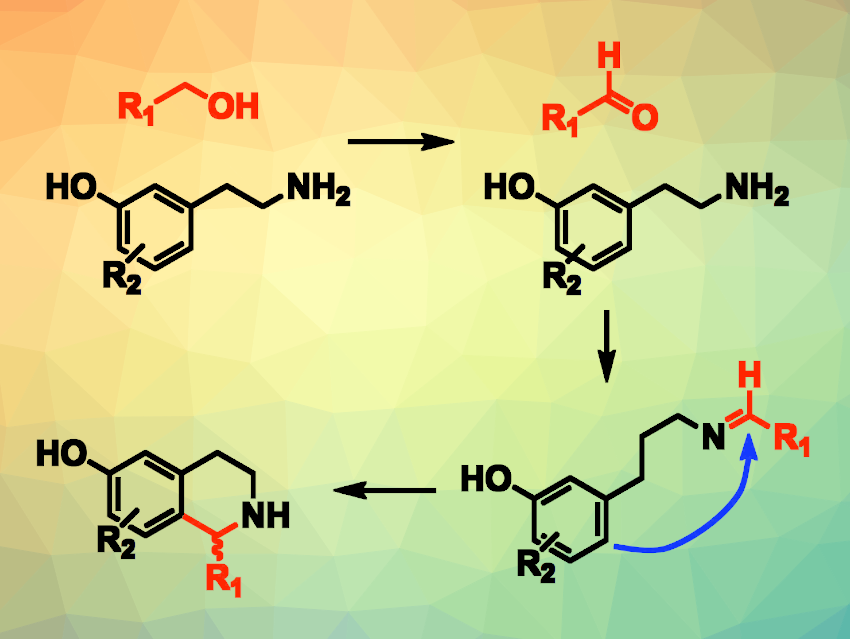
Dylan W. Domaille, Colorado School of Mines, Golden, USA, and colleagues have developed a way to combine a live-cell biocatalyzed alcohol oxidation with a chemocatalyzed Pictet-Spengler reaction (pictured). The team used live Gluconobacter oxydans bacteria or Komagataella pastoris yeast to convert C2-C5 alcohols to aldehydes (pictured in red), which undergo a condensation with m-tyramine or tryptamine substrates to form imine intermediates.
A phosphate-catalyzed Pictet-Spengler annulation then gives tetrahydroisoquinoline alkaloids or tryptolines with high regioselectivity. This reaction is biocompatible, allowing for a two-step, one-pot cascade reaction.
Combining a biocompatible chemocatalyzed transformation with a mild biocatalytic alcohol oxidation, unnatural N-heterocycles were produced in aqueous conditions at mild temperatures (28 °C) under ambient atmosphere. According to the team, the methodology could be expanded to kinetic resolution for the synthesis of chiral products.
- Interfacing Whole Cell Biocatalysis with a Biocompatible Pictet‐Spengler Reaction for One‐Pot Syntheses of Tetrahydroisoquinolines and Tryptolines,
Campbell Andersen, Luke D. Knudson, Dylan W. Domaille,
ChemBioChem 2023.
https://doi.org/10.1002/cbic.202300464