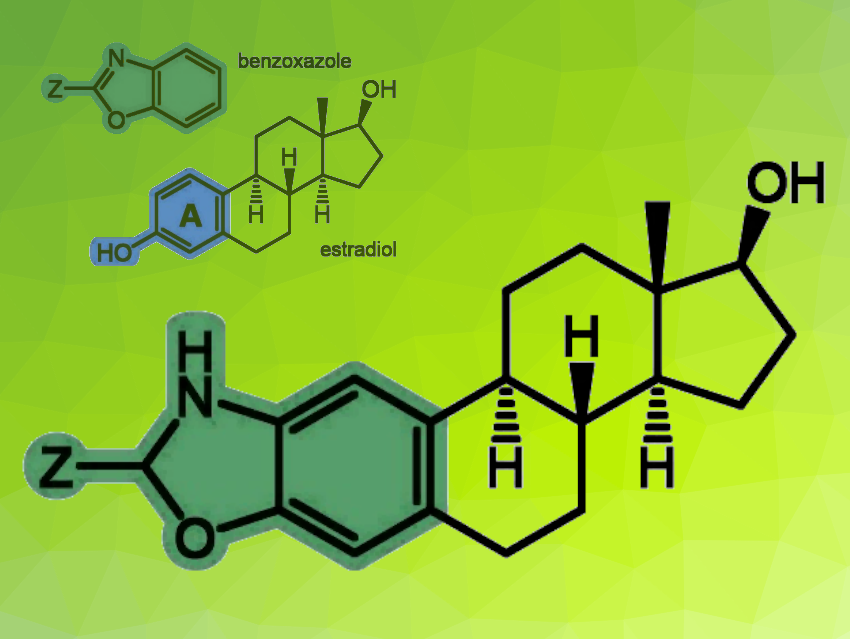
Estradiol is the major female sex hormone. It has a tetracyclic skeleton (pictured), with the A-ring being aromatic. In addition to its physiological functions, it also plays a crucial role in hormone-dependent cancers by stimulating cell proliferation via estrogen receptors. Some estradiol derivatives that are chemically modified at the A-ring—such as 2-methoxyestradiol—no longer have an estrogenic effect and can have anticancer effects.
György T. Balogh, Semmelweis University, Budapest, Hungary, and Budapest University of Technology and Economics, Hungary, Éva Frank, University of Szeged, Hungary, and colleagues have synthesized hybrid molecules from estradiol-derived precursors in which a benzoxazole unit is incorporated into the A-ring of the framework (pictured). Benzoxazoles have diverse pharmacological activities.
The team used 2-amino-estradiol and its 17β-acetate to prepare estradiol-benzoxazole hybrids. Some derivatives were synthesized from these compounds by direct cyclization, while additional 2-substituted analogues were accessed by functional group interconversions. The researchers obtained a compound library of 32 structurally related analogs.
Pharmacological studies showed that some of the derivatives have potent and selective in vitro anticancer activity and can induce apoptosis (programmed cell death) in human cancer cells, similar to 2-methoxyestradiol. A few derivatives were identified as primary candidates for further drug development. According to the researchers, future investigations of these selected compounds may provide a better understanding of their mechanism of action.
- Medicinal‐Chemistry‐Driven Approach to 2‐Substituted Benzoxazole–Estradiol Chimeras: Synthesis, Anticancer Activity, and Early ADME Profile,
Ferenc Kovács, Ildikó Huliák, Hédi Árva, Mónika Kiricsi, Dóra Erdős, Marianna Kocsis, Gergely Takács, György T. Balogh, Éva Frank,
ChemMedChem 2023.
https://doi.org/10.1002/cmdc.202300352