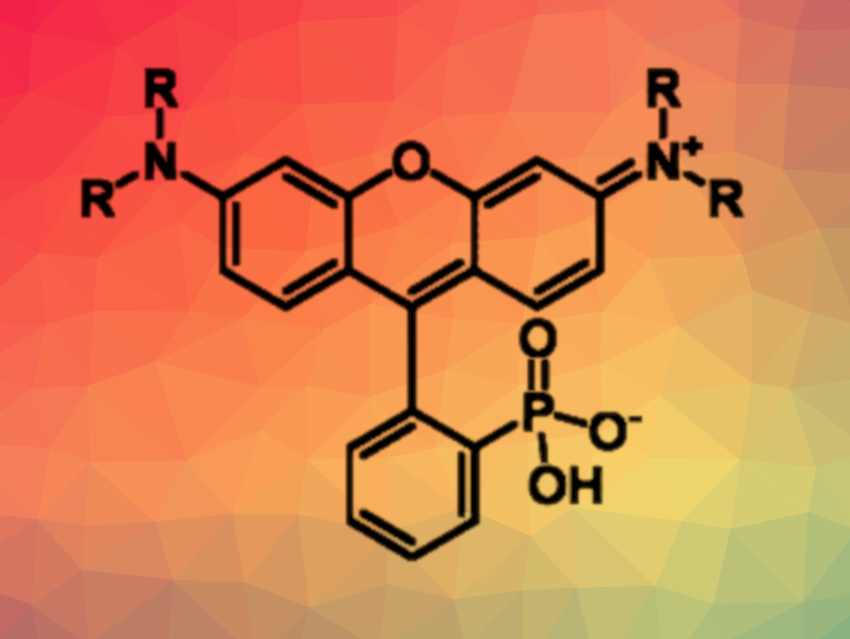
Rhodamine dyes are widely used as fluorophores in bioimaging applications. The functionalization of these dyes can change their properties—such as absorption and emission wavelengths, brightness, solubility, and stability—significantly. For fluoresceins, for example, which are similar to rhodamines, phosphonate substitution at the 3-position of the pendant aryl ring can lead to improved water solubility. The 3-phosphonate substitution of rhodamines could also be useful. However, there is a lack of general synthetic approaches for the 3-substitution of these compounds.
Evan W. Miller, University of California, Berkeley, USA, and colleagues have developed a scalable synthesis of 3-phosphonorhodamines under mild conditions. The approach is based on a condensation of aminophenols and 2-phosphonobenzaldehyde. The team reacted 2-phosphonobenzaldehyde with a series of aminophenols in 2,2,2-trifluoroethanol (TFE) at 80 °C to obtain triarylmethanes. These intermediates then underwent dehydration and oxidation to the corresponding 3-phosphonorhodamines, e.g., by using p-chloranil as an oxidant in methanol or by exposure to air in methanol or dimethyl sulfoxide (DMSO). Aminophenols with phosphonate monoesters can also be used in the initial condensation, providing access to ester analogues.
Using this approach, the desired 3-phosphonorhodamines can be synthesized in high yields on a 1.5 mmol scale, often without a need for chromatography. The products have optical properties that are very similar to 3-carboxy- and 3-sulfono analogues, but a much higher water solubility, and are useful for cellular imaging.
- Mild and scalable synthesis of phosphonorhodamines,
Joshua L. Turnbull, Ryan P. Golden, Brittany R. Benlian, Katharine M. Henn, Soren M. Lipman, Evan W. Miller,
Chem. Sci. 2023.
https://doi.org/10.1039/D3SC02590J