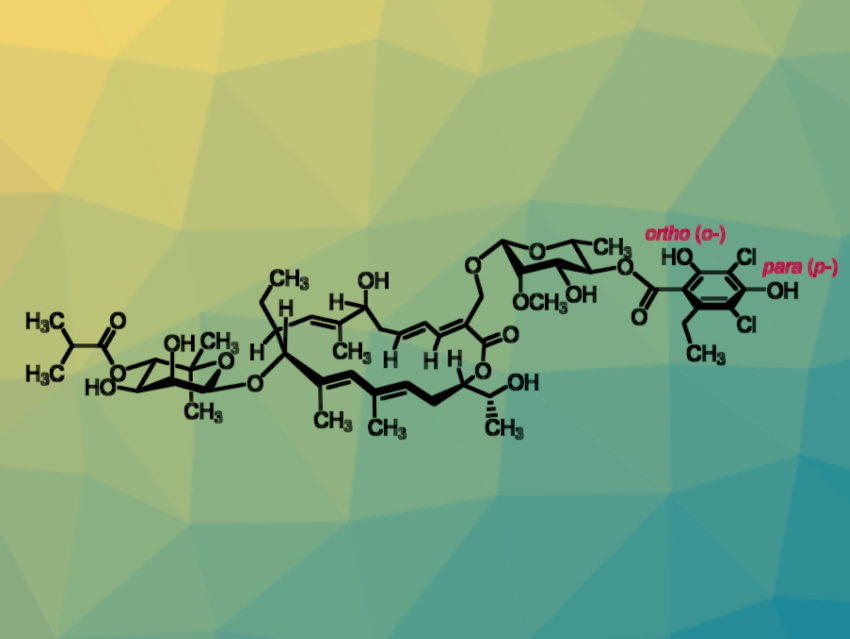
The antibiotic fidaxomicin (pictured) has been used to treat Clostridioides difficile infections for about two decades. Recently, a first case of fidaxomicin resistance was observed. Due to the complex structure of the compound, the development of next-generation fidaxomicin antibiotics to combat resistance is challenging.
Karl Gademann, University of Zurich, Switzerland, and colleagues have developed a protocol for the synthesis of previously inaccessible fidaxomicin derivatives. The aromatic moiety of fidaxomicin contains two phenolic groups that are potential functionalization handles. Selectively protecting the more reactive group of the two using an allyl protecting group allowed the team to functionalize the compound in the less reactive ortho-position. They performed an alkylation followed by a palladium-catalyzed deprotection.
The researchers measured the activity of the newly obtained derivatives against C. difficile and Mycobacterium tuberculosis. The new ortho-functionalized derivatives were more potent against M. tuberculosis than known para– or bis-substituted analogues, as was predicted from the binding mode of fidaxomicin.
In addition, for the first time, a fidaxomicin derivative with activity against the Gram-negative model organism Caulobacter crescentus was identified. According to the researchers, this demonstrates that expanding the spectrum of activity of fidaxomicin to new bacterial species is possible. Further development could lead to next-generation fidaxomicin antibiotics that overcome resistance and offer new treatment options.
- Phenolic Substitution in Fidaxomicin: A Semisynthetic Approach to Antibiotic Activity Across Species,
Erik Jung, Anastassia Kraimps, Silvia Dittmann, Tizian Griesser, Jordan Costafrolaz, Yves Mattenberger, Simon Jurt, Patrick H. Viollier, Peter Sander, Susanne Sievers, Karl Gademann,
ChemBioChem 2023.
https://doi.org/10.1002/cbic.202300570