Dibenzofurans are often found in natural products or biologically active compounds. Many contain electron-rich aryl rings. The central five-membered ring of the dibenzofuran skeleton can be closed via C–C bond formation from a diarylether or via C–O bond formation from a biaryl precursor. Forming the key intramolecular C–C bond via C–H functionalization is an interesting approach, but can be challenging for electron-rich substrates.
Gerard P. McGlacken, University College Cork, Ireland, and colleagues have developed an approach for the synthesis of electron-rich dibenzofurans based on a Pd-catalyzed C–H functionalization (pictured below). Generally, such Pd-mediated catalytic transformations require a base, added ligands, and a solvent, but the team used tetraalkylammonium salts, particularly NBu4OAc, which can act as solvent, ligand, and base in the reaction, together with Pd(OAc)2 as the catalyst. The reactions were performed at 125 °C.
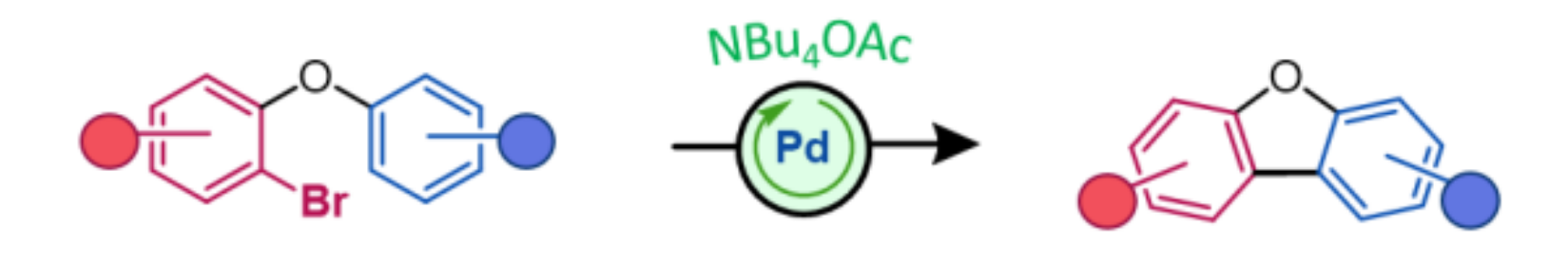
The team obtained a range of dibenzofurans, in particular, difficult-to-access electron-rich examples, in good to excellent yields. Overall, the process provides a simple, efficient, and green route to electron-rich dibenzofurans.
- Access to Electron‐Rich Dibenzofurans through NBu4OAc Mediated Palladium Catalysis,
Mark Power, Katrina Mackey, Mark Light, David Jones, Gerard P. McGlacken,
Eur. J. Org. Chem. 2023.
https://doi.org/10.1002/ejoc.202300790