Transmetalations occur commonly in catalytic organic reactions, e.g., in the case of the Negishi cross-coupling, which features a Zn-to-Pd transmetalation step from an organozinc nucleophile to the catalyst. A transmetalation that goes the other way, i.e., from the catalyst to a metal such as zinc, could lead to useful organometallic intermediates. The transmetalation from Co(II) to Zn, for example, has favorable thermodynamics.
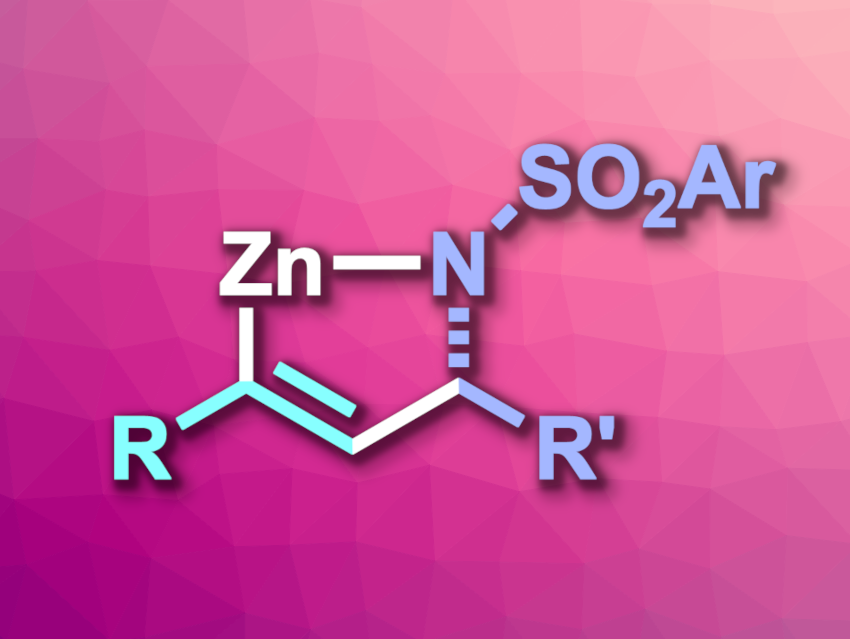
Christopher Uyeda, Purdue University, West Lafayette, IN, USA, and colleagues have developed an asymmetric synthesis of Zn metallacycles (general product structure pictured) that involves such a catalyst-to-Zn transmetalation. The team reacted a range of terminal alkynes with imines that feature different aryl or heteroaryl substituents in the presence of CoCl2 as a catalyst, together with a chiral phosphinooxazoline (Phox)-type ligand, and elemental zinc. The reactions were performed in dimethylacetamide (DMA) at room temperature.
After an acidic workup, the reaction gave allylic amines as coupling products—enantioselectively and in good yields. When the team analyzed the reaction mixture before the workup, they found that organozinc species were formed as intermediates. These intermediates can be used directly in a variety of transformations, e.g., halogenations, chalcogenations, alkylations, or arylations, providing access to trisubstituted allylic amines. The team proposes a reaction mechanism for the formation of Zn metallacycles that involves the oxidative coupling of the alkyne and imine reaction partners at the active cobalt catalyst to form a Co metallacycle, which then undergoes transmetalation to zinc.
- Catalytic Asymmetric Synthesis of Zinc Metallacycles,
Hayden D. Bishop, Qiang Zhao, Christopher Uyeda,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c05885