The selective oxygenation of C(sp3)–H bonds is a useful approach to synthesizing functionalized organic molecules. However, it can be challenging to selectively oxidize a certain C(sp3)–H bond to give an alcohol, and the alcohol products can be prone to further oxidation. This affects, for example, the selective benzylic oxidation of alkylarenes to produce benzyl alcohols.
Hai-Chao Xu, Xiamen University, China, and colleagues have developed a practical and cost-efficient electrochemical method for the selective monooxygenation of benzylic C(sp3)–H bonds using continuous flow reactors. The electrochemical reactions produce trifluoroacetate esters that are resistant to further oxidation but undergo facile hydrolysis during aqueous workup to form benzylic alcohols (pictured below).
The team used a variety of alkylarenes as substrates and reacted them with CF3CO2H in the presence of 2,6-lutidine with MeCN or CH2Cl2/HFIP (HFIP = 1,1,1,3,3,3-hexafluoro-2-propanol) as the solvent. They employed a single-pass flow cell with a graphite anode and a Pt cathode. After pumping the reaction solution through the flow cell, it was treated with aqueous NaHCO3.
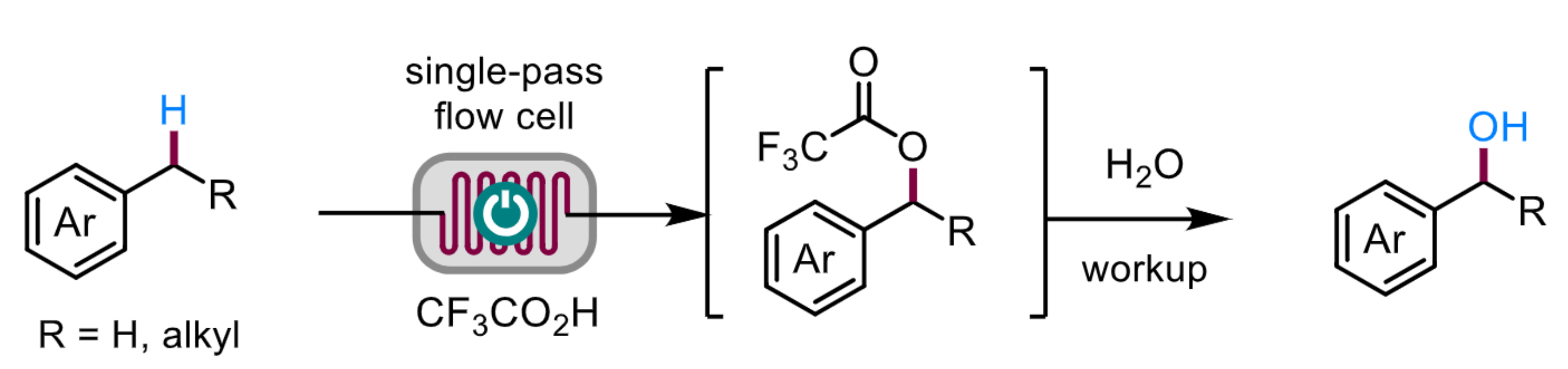
The desired benzylic alcohols were obtained in moderate to high yields. The method has a broad substrate scope and high site selectivity. It requires no catalysts or chemical oxidants. Using twenty parallel flow cells, the team produced 115 g of one of the alcohol products, demonstrating the scalability of the approach.
- Continuous Flow Electrochemistry Enables Practical and Site‐Selective C–H Oxidation,
Tian-Sheng Chen, Hao Long, Yuxing Gao, Hai-Chao Xu,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202310138



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)