The hydrosilylation of imines has become a promising strategy to synthesize a wide range of valuable compounds, including amines, amino acids, and pharmaceutical intermediates. Traditionally, a diverse range of transition metal complexes have been used to facilitate this transformation. However, there are very few main-group compounds that can activate imines.
Aluminum complexes have brought significant advancements to main group catalytic systems and proven valuable in reducing various functional groups, such as alkenes, alkynes, carbonyls, and CO2. However, exploration of the hydrosilylation of imines with Al catalysts remains limited.
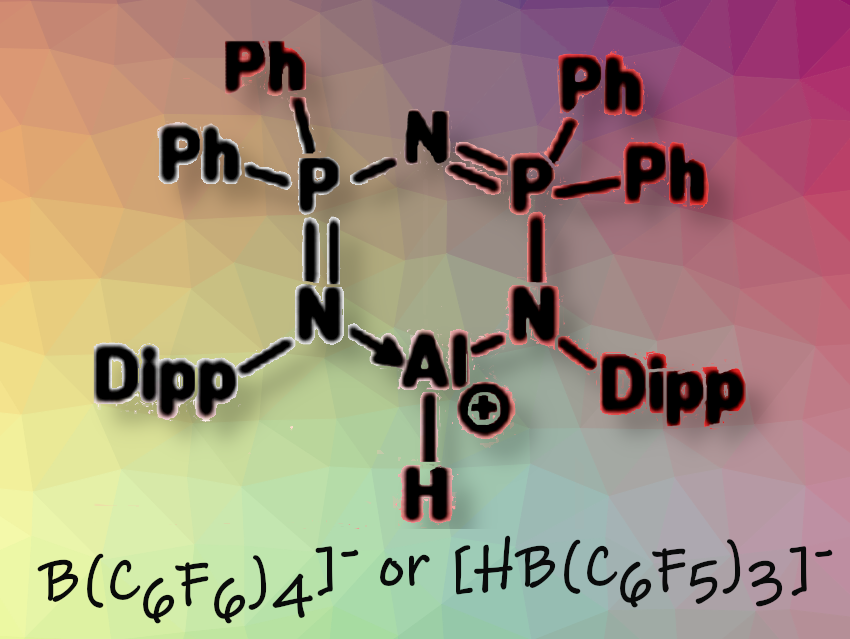
Sanjay Singh and colleagues, Indian Institute of Science Education and Research in Mohali, Punjab, India, have used bis(phosphinimino)amide-functionalized, three-coordinated Lewis acid cationic aluminum hydrides to enable imine hydrosilylation. To study the impact of steric bulk, they examined various silanes, including phenylsilane, triethylsilane, methylphenylsilane, tetramethyldisiloxane (TMDSO), and polymethylhydroxysiloxane (PHMS). The team isolated the amines in respectable yields.
The catalytic process showed excellent tolerance toward different functional groups. The researchers have conducted a series of controlled experiments to investigate the underlying mechanistic pathway. They concluded that a Lewis adduct forms between the cationic aluminum center and the imine nitrogen. This adduct then reacts with the silane to produce the final product.
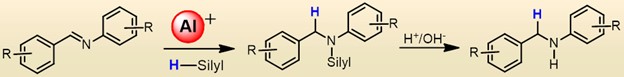
According to the researchers, this work sheds light on the reactivity of cationic aluminum complexes. The findings offer insights into understanding the catalytic role of discrete cationic aluminum complexes and will provide guidance for designing more efficient and selective catalytic systems.
- Catalytic Hydrosilylation of Imines by Aluminum Hydride Cations,
Mamta Bhandari, Sandeep Rawat, Mandeep Kaur, Sanjay Singh,
European Journal of Organic Chemistry 2023.
https://doi.org/10.1002/ejoc.202300674