With their tunable σ-donor/π-acceptor properties, olefin ligands can be useful in various fields of research, e.g., (asymmetric) catalysis. The incorporation of (heavy) main group elements into olefin ligands can modify their properties like denticity, bite angle, and the shape/energy of their frontier orbitals. This could ideally lead to enhanced characteristics of the respective corresponding metal complexes.
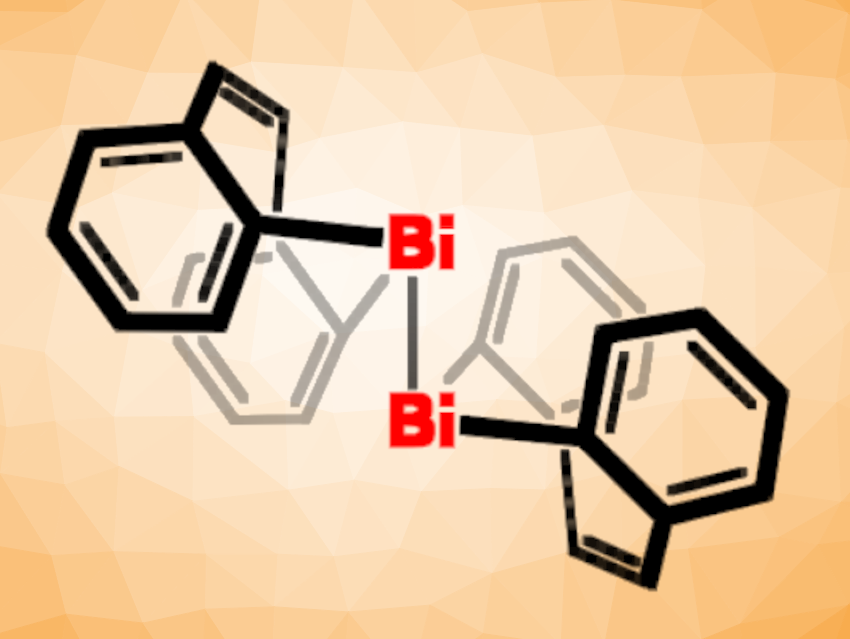
Crispin Lichtenberg, University of Marburg, Germany, and colleagues have synthesized bis[dibenzobismepine], a dibismuthane (R2Bi–BiR2) with two olefin moieties (pictured above). The dibismuthane product was prepared from an iodine-substituted bismepine.
The reactive Bi–Bi bond in this dibismuthane was further used to synthesize two series of chalcogen-bismuth compounds, R2Bi–EPh and R2Bi–E–BiR2 (latter pictured below, E = O–Te). A reaction with diphenyl dichalcogenides of the type PhE−EPh gave R2Bi–EPh, while a reaction with elemental sulfur, selenium, or tellurium led to complexes of the type R2Bi–E–BiR2.
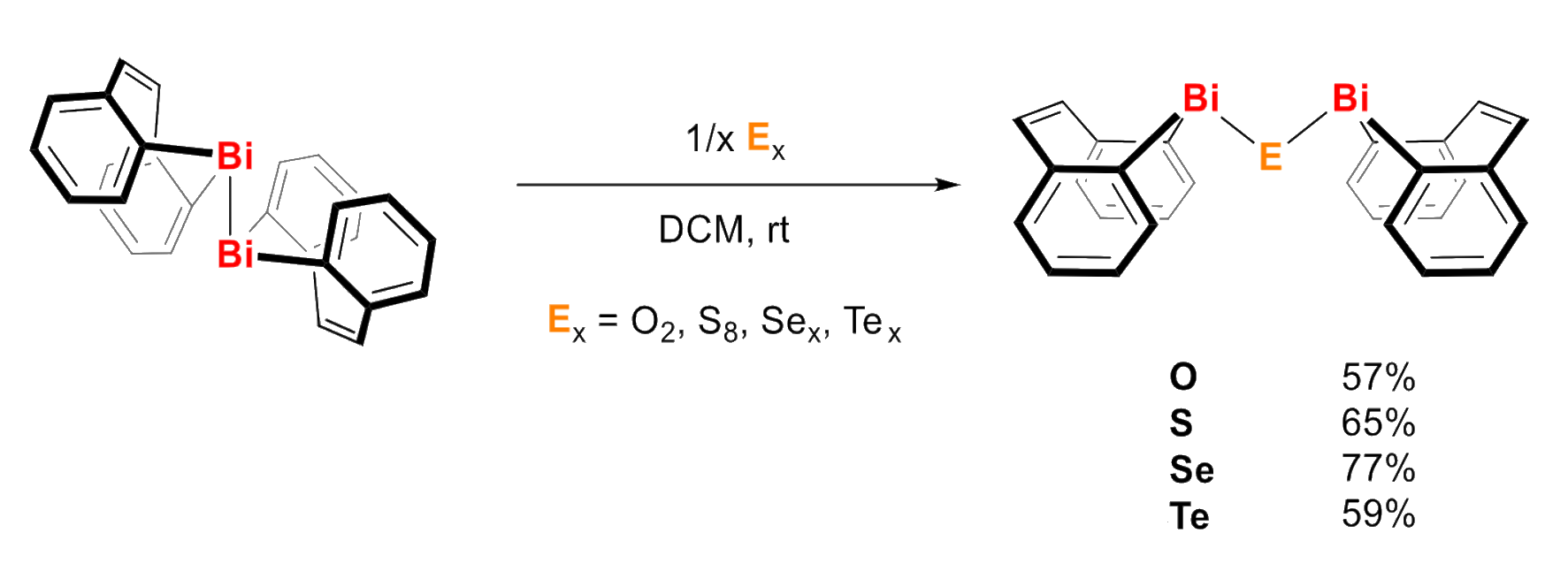
Using density functional theory (DFT) calculations, the team identified compounds of the type R2Bi–E–BiR2 as potential hybrid olefin/chalcogen ligands towards electron-rich transition metals. The sulfur species was predicted to be a privileged ligand due to a favorable combination of bond lengths and angles in the Bi–S–Bi unit. These results could be helpful for the utilization of heavy main group elements for the precise fine-tuning of ligand properties.
- A Dibismuthane with Olefin Functional Groups: towards Tridentate Hybrid Chalcogen/Olefin Ligands,
Felix Geist, Sebastián Martínez, Jacqueline Ramler, Kai Oberdorf, Lisa Brändler, Sascha Reith, Crispin Lichtenberg,
Eur. J. Inorg. Chem. 2023.
https://doi.org/10.1002/ejic.202300415