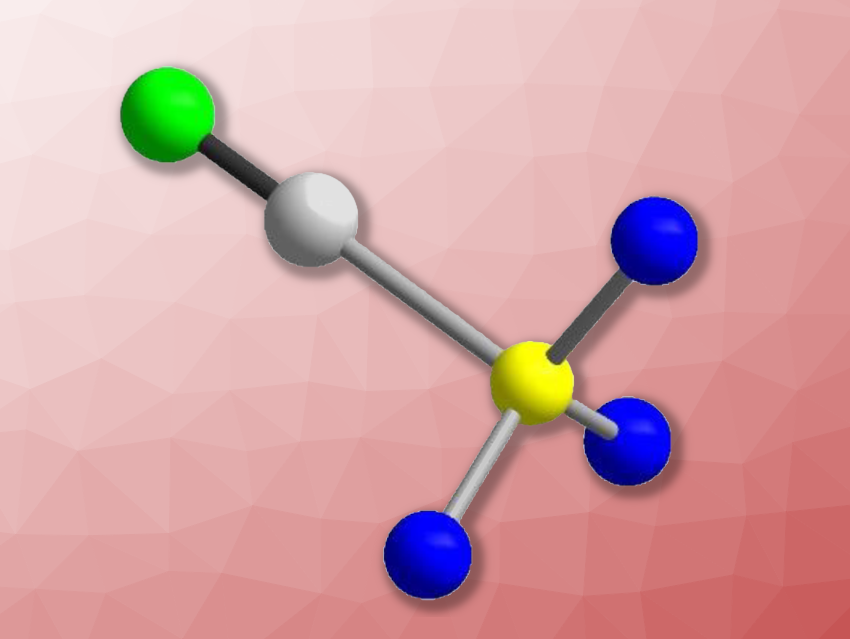
Sulfur trioxide (SO3) is a strong oxidant and a Lewis acid. There is a limited number of known Lewis acid-base complexes of SO3, some of which can be used for the synthesis of sulfuric acid derivatives. Simple sulfate derivatives such as the fluoro- and the chloro-sulfate anion ([SO3F]– and [SO3Cl]–), can be considered Lewis acid-base complexes of SO3 with F– and Cl–, respectively. The pseudo-halide analogue of the fluorido- and chlorido-sulfates [SO3CN]– has been considered unstable so far.
Mathias Wickleder, University of Cologne, Germany, and colleagues have found a suitable route to stabilize and synthesize the [SO3CN]– ion for the first time. The approach is based on the reaction of a suitable cyanide with the pyridine-sulfur trioxide complex py∙SO3. The team chose a TBA salt (TBA = tetra-n-butyl-ammonium, [N(C4H9)4]+) as the cyanide source and found that the reaction of TBA(CN) and py∙SO3 in 1,4-dioxane at room temperature gives single crystals of [N(C4H9)4][SO3CN].
The product was analyzed using X-ray diffraction (structure of the [SO3CN]– anion pictured) and vibrational spectroscopy. The developed approach could also be suitable for the preparation of other, hitherto unknown pseudohalido sulfates. In addition, [N(C4H9)4][SO3CN] is soluble in organic solvents and could be a suitable starting material for the synthesis of related salts.
- The cyanido‐sulfate anion [SO3CN]–,
Thomas Kasperowicz, David van Gerven, Mathias Wickleder,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202301761