Oxidative alkene cleavage is a widely used reaction for introducing oxygen-functionalities such as acids, carbonyls, and alcohols into complex molecules. The synthesis of various bioactive compounds involves the cleavage of alkenes as a key synthetic step.
Among the arsenal of available oxidation methods, ozonolysis is a useful approach, but it requires specialized equipment and can be hazardous due to the formation of explosive ozonide intermediates. Using enzymes can be a promising alternative with safe and mild reaction conditions as well as the possibility to functionalize complex compounds in a regio-, chemo-, and stereo-specific fashion.
Martina L. Contente, University of Milan, italy and colleagues have used a dioxygenase from Caulobacter segnis (called CsO2) as a selective and efficient biocatalyst for the cleavage of the C=C double bonds of various natural and synthetic trans-stilbenes (pictured). Thus, the team has developed a green, enzymatic, ozonolysis-like reaction.
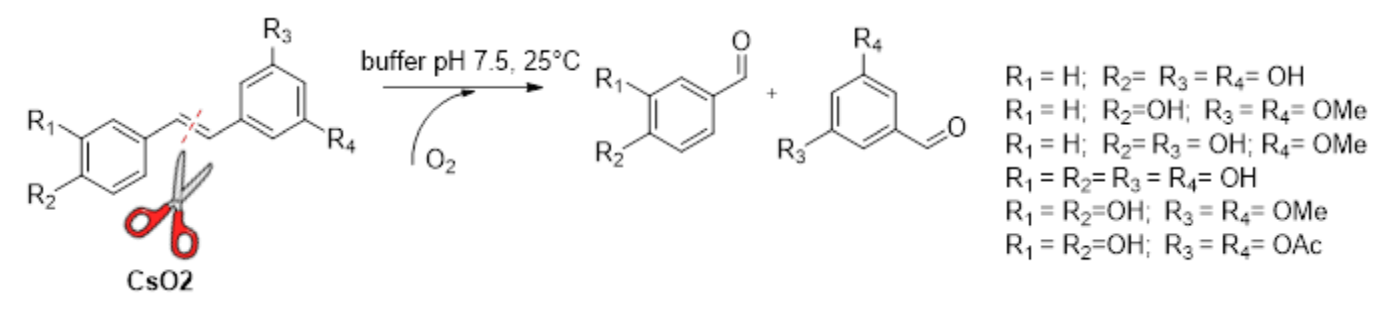
The researchers found that a hydroxyl group was necessary in the para-position of the phenyl group (such as in resveratrol and its derivatives) for the reaction to take place, which provides insights into substrate recognition. Under optimized conditions, the biotransformation of resveratrol, used as model substrate, was successfully carried out on the 50-mL scale, which confirms the possibilty of using the developed biocatalytic system as a preparative method.
- Caulobacter segnis dioxygenase CsO2: a practical biocatalyst for stilbenoid ozonolysis,
Valerio De Vitis, Pietro Cannazza, Luce Mattio, Diego Romano, Andrea Pinto, Francesco Molinari, Tommaso Laurenzi, Ivano Eberini, Martina Letizia Contente,
ChemBioChem 2023.
https://doi.org/10.1002/cbic.202300477