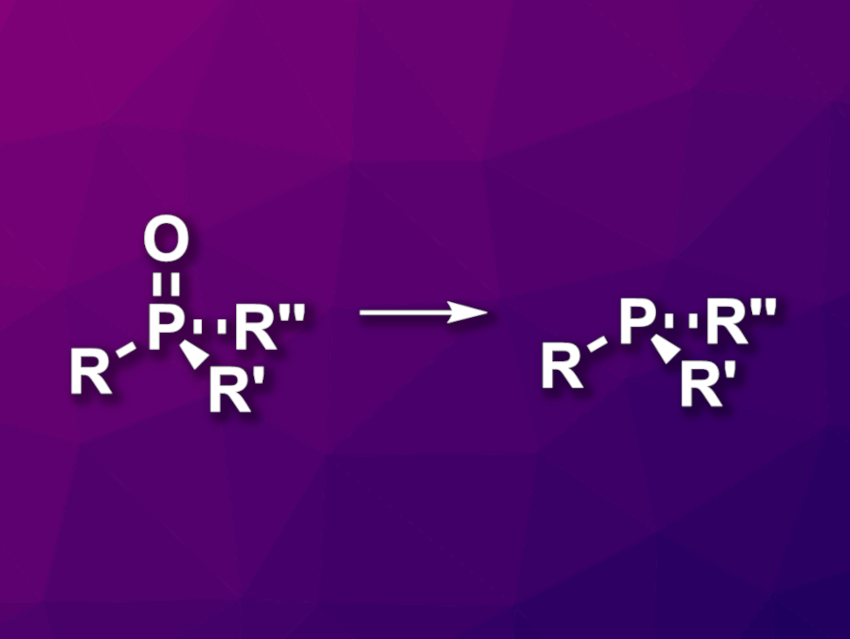
Phosphine oxides are often produced as waste products in chemical transformations. Reducing them to phosphines can help to reduce waste and reuse phosphorus resources. The development of a cost-effective method for the reduction of phosphine oxides under mild conditions is, thus, an interesting research target.
Le Li, Sun Yat-sen University, Guangzhou, China, and colleagues have developed an approach to the reduction of phosphine oxides to phosphines (general reaction pictured) that is mediated by N,N,N’,N’-tetramethylethylenediamine (TMEDA). The team reduced a variety of triaryl phosphine oxides and several phosphine oxides with alkyl substituents, using oxalyl chloride to create a chlorophosphonium salt (CPS) intermediate, sodium iodide to convert this intermediate to an iodine-containing species, and TMEDA to achieve a hydride transfer.
The reactions were performed in MeCN at 60 °C. The desired phosphines were obtained in moderate to excellent yields. According to the researchers, the work shows that tertiary amines can serve as mild and cost-effective organic reductants for the reduction of phosphine oxides.
- Tertiary Amine-Mediated Reductions of Phosphine Oxides to Phosphines,
Keshu Yin, Mingjie Wei, Zhenguo Wang, Wenjun Luo, Le Li,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c01690
![Cucurbit[7]uril-Based Polymers with Dual Photochromism and Fluorescence](https://www.chemistryviews.org/wp-content/uploads/2025/09/70145-125x94.png)
