The addition of fluorine to organic molecules can be useful to tune their properties, e.g., for applications in pharmaceutical chemistry. Terminal monofluoroalkenes, for example, can be useful in organic synthesis, materials science, and drug development. Existing approaches to their synthesis can have drawbacks such as low selectivity, limited substrate scopes, or insufficient scalability.
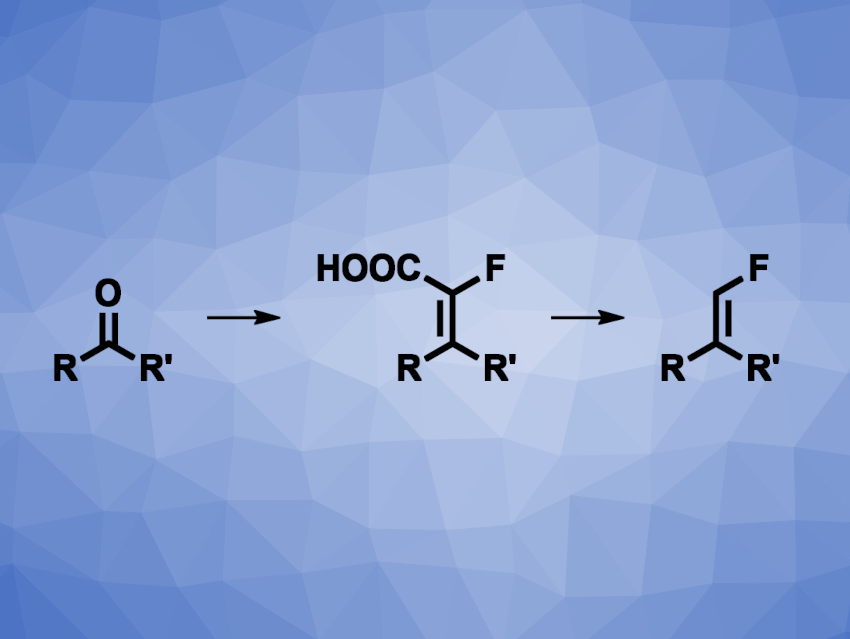
Ivan Volchkov, Jonathan T. Reeves, Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA, and colleagues have developed a practical method for the synthesis of di- and trisubstituted terminal monofluoroalkenes (vinyl fluorides). The team’s approach is based on a selective Horner–Wadsworth–Emmons olefination of ketones or aldehydes, hydrolysis to α-fluoroacrylic acids, and decarboxylation (general reaction sequence pictured). They used triethyl-2-fluoro-2-phosphonoacetate for olefinations with E-selectivity. MeMgBr served as a base for ketone substrates, and n-BuLi was used for aldehydes.
The resulting esters were hydrolyzed using LiOH, giving α-fluoroacrylic acids. These acids can crystallize in many cases, which eliminates the need for chromatography for product purification. To obtain the desired vinyl fluorides, the team performed decarboxylations using AgOAc as a catalyst and N-methyl-2-pyrrolidinone (NMP) as the solvent. Overall, the work provides convenient access to α-fluoroacrylic acids and terminal di- and trisubstituted vinyl fluorides.
- Practical Synthesis of Terminal Vinyl Fluorides,
Ivan Volchkov, Brent V. Powell, Olga V. Zatolochnaya, Joyce C. Leung, Scott Pennino, Lifen Wu, Nina C. Gonnella, Bangaru Bhaskararao, Marisa C. Kozlowski, Jonathan T. Reeves,
J. Org. Chem. 2023.
https://doi.org/10.1021/acs.joc.3c00917



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)